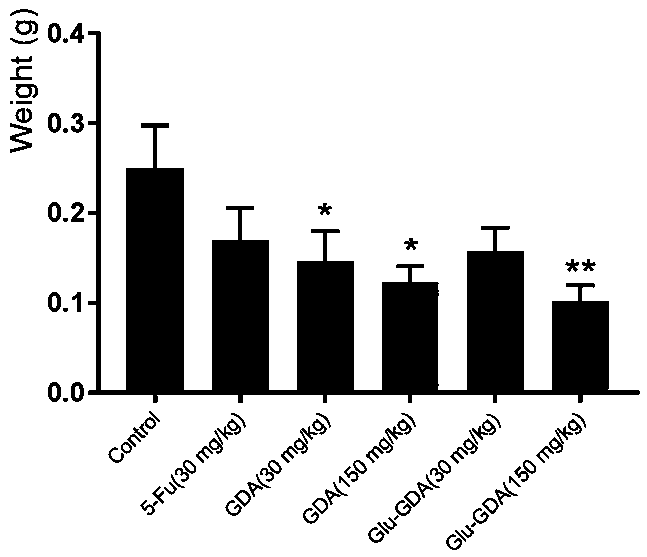Triterpenoid compound, preparation method thereof and application thereof in preparing anticancer drugs
A technology of anticancer drugs and compounds, applied in the directions of steroids, chemical instruments and methods, antitumor drugs, etc., can solve the problems of few types and unsatisfactory anticancer activity, and achieve the inhibition of volume growth and great medicinal value. , non-toxic side effects and drug dependence effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The raw material medicine of the present invention is the fruit body of Ganoderma lucidum produced in Hainan, and the fruit body is chopped and ground for extraction.
[0040] The ethanol extraction method adopted in the preparation method of the present invention can be percolation method, dipping method or reflux extraction method. As a preference, the preparation method of the present invention preferably uses ethanol reflux to extract the raw material drug, and the operation steps are further preferably:
[0041]Use 50-100% ethanol, more preferably 95% ethanol, which is 5-7 times the total weight of the raw material drug, and reflux the raw material drug for 3-4 times, 60 min each time. Below 60°C, the filtrate obtained after ethanol extraction is concentrated into a thick paste of crude extract. The crude extract was subjected to normal phase silica gel chromatography (CHCl 3 / MeOH 9:1-3:1, v / v) after gradient elution, point plate and combine samples to obtain Fra...
Embodiment 2
[0052] The present invention provides a compound 26-O-β-D-glucopyranosyl-Ganodecalone A (Ganodecalone A has poor water solubility), which has good anticancer activity and excellent solubility, and is more suitable for preparing water-soluble anticancer drugs. Its structure is as follows:
[0053]
[0054] Its synthesis method is as follows:
[0055]
[0056] (a) TMSOTf,CH 2 Cl 2 ,-30℃, 89%; (b)CH 3 ONa,CH 3 OH:CH 2 Cl 2 =1:1,95%.
[0057] Under argon protection, compound 1, Ganodecalone A (91 mg, 0.20 mmol) and 2,3,4,6-tetra-acetyl-α-D-glucopyranose trichloroimidate donor 2 (280 mg , 0.26mmol, 1.30equiv.) dissolved in 4mL of anhydrous CH 2 Cl 2 to which was added the dry Molecular sieves (300mg), stirred at -30°C for 30min, added trifluoromethylsilyl trifluoromethanesulfonate (TMSOTf) (7μL, 0.04mmol, 0.2equiv.), stirred at -30°C for 4h, TLC showed that the reaction was complete, Triethylamine quenched the reaction, filtered, concentrated, and flash column chro...
Embodiment 3
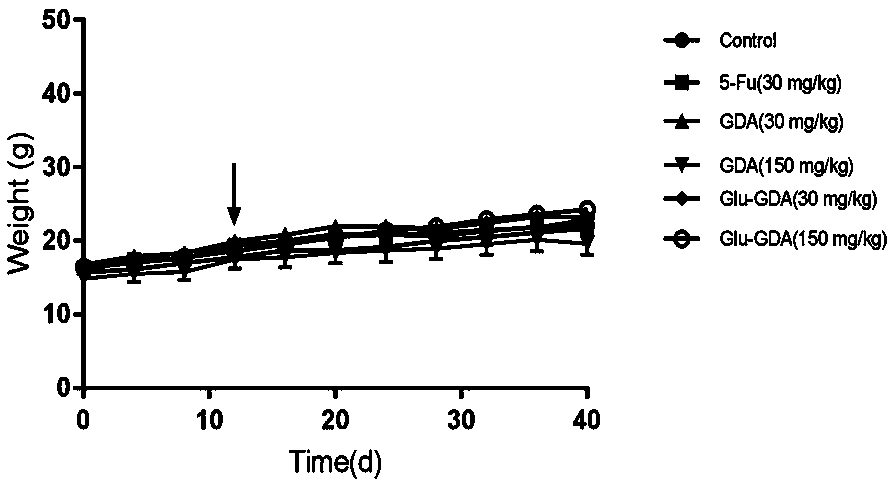
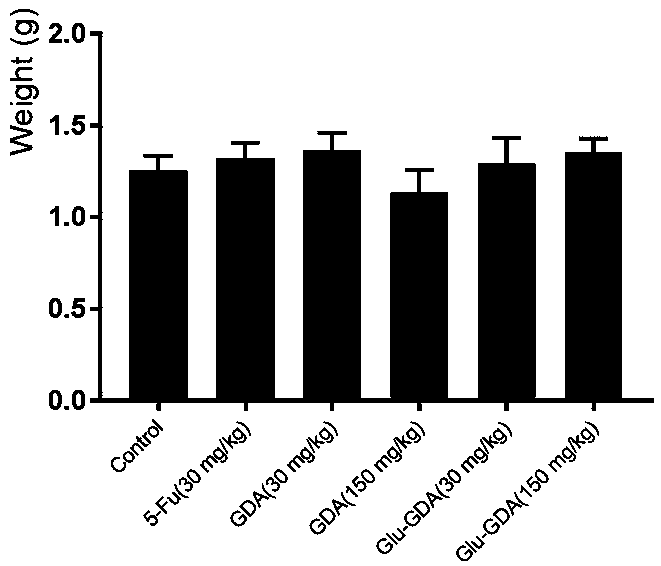
[0061] Growth inhibitory activity of compounds Ganodecalone A and 26-O-β-D-glucopyranosyl-Ganodecalone A on tumor cells in vitro.
[0062] The detection method is as follows:
[0063] (1) Cell plating
[0064] The cells were subcultured, and when the cell fusion rate reached about 80%, the old medium was discarded and washed with phosphate buffered saline (PBS, pH7.4) to remove residues, and then 1 mL of 0.5‰ (w / v) trypsin was added to decompose the cells. Digestion, when about 80% of the cells become round, add serum-containing medium to stop digestion, blow the wall of the suction bottle with a pipette gun, blow off the adherent cells as much as possible, add the cell suspension to the centrifuge tube and place it in the centrifuge , centrifuged at 1000rpm for 5min, discarded the supernatant, resuspended the cells with fresh medium, blown and sucked with a pipette gun to disperse the cells as much as possible, counted, and diluted the cell suspension to 5×104 cells / mL. Add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com