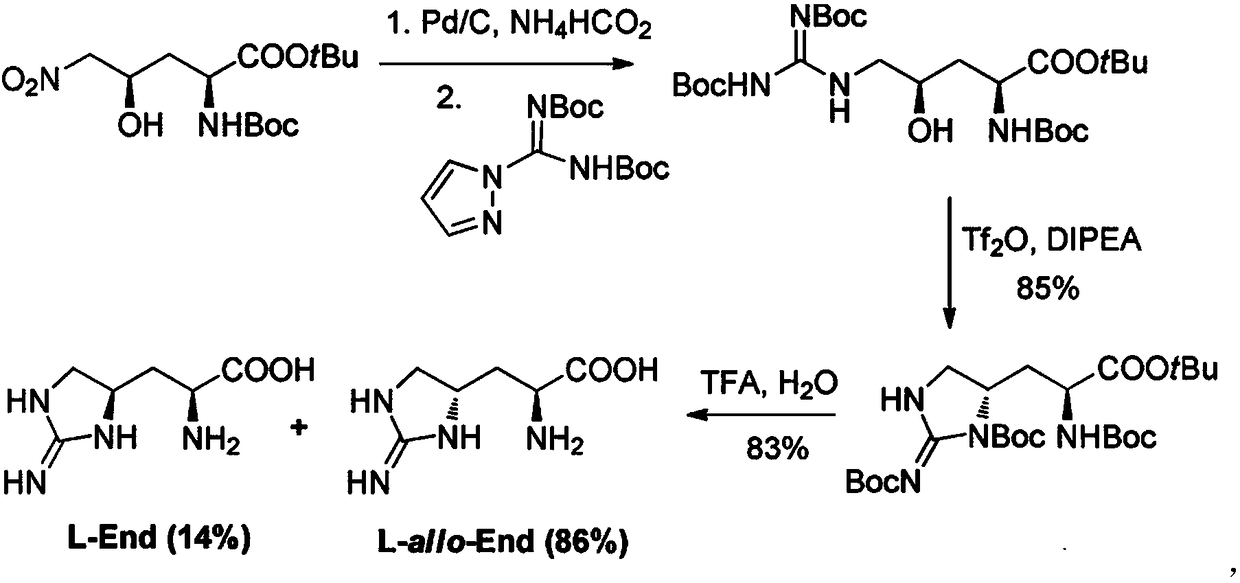
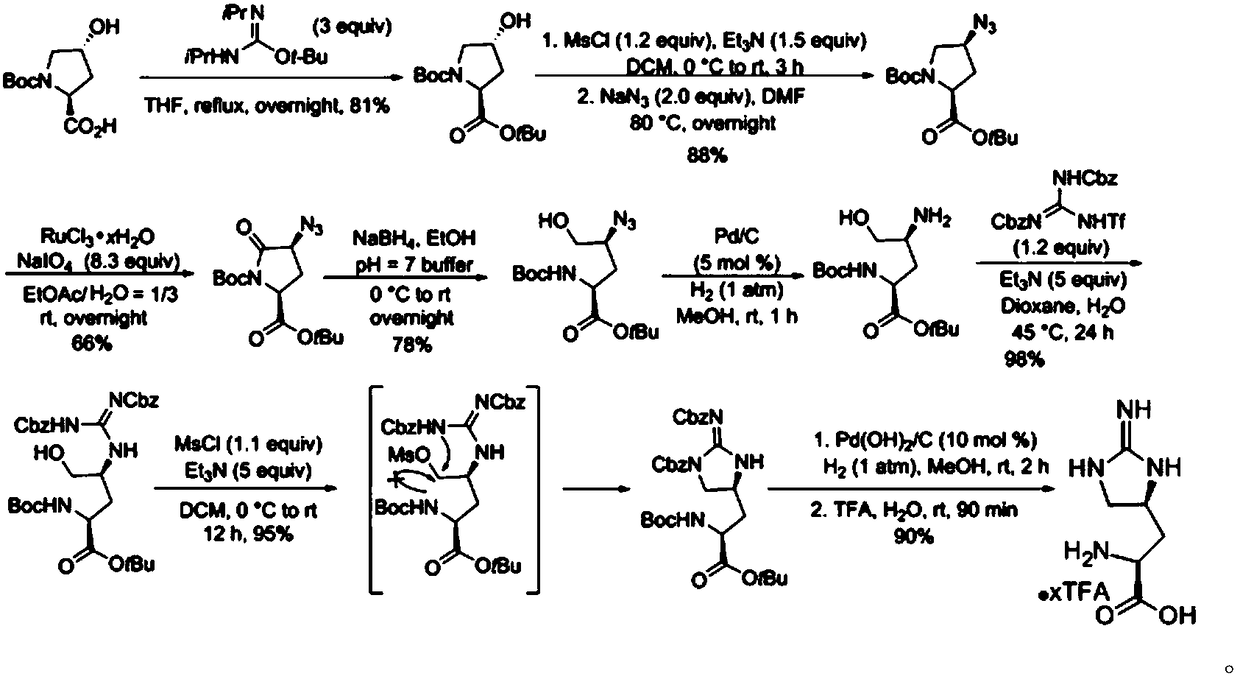
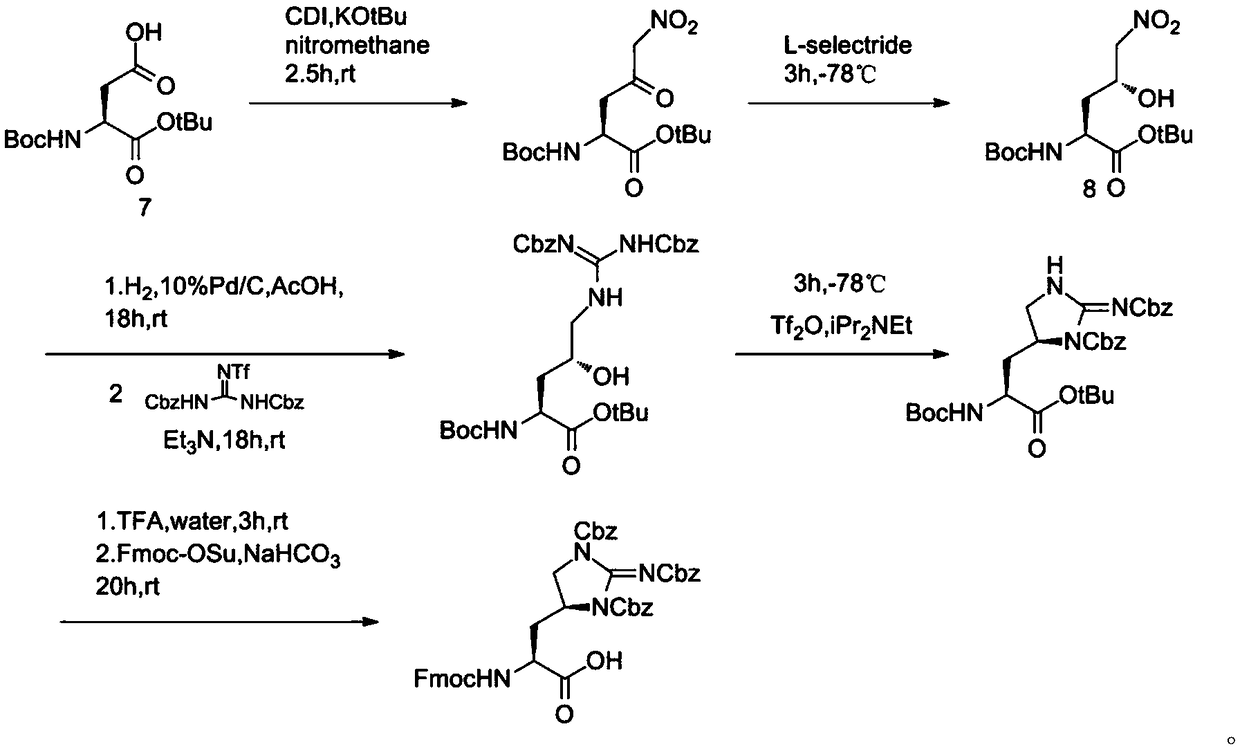
Synthetic route for antibiotic teixobactin and analogs thereof
A solid-phase synthesis, fmoc-l-technology, applied in the preparation method of peptides, organic chemistry, peptides, etc., can solve the problems of difficulty in the total synthesis of teixobactin, complex structure, etc., and achieve the effect of less by-products and high esterification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143]
[0144] Synthesis of compound shown in formula 2:
[0145] Add the compound (10mmol) shown in Formula 1 into a round bottom flask, add 20ml of acetonitrile, add solid sodium bicarbonate (40mmol) and stir at zero, slowly add elemental iodine (30mmol) and stir for one hour, then spin dry the acetonitrile; Next, add acetic acid and methanol mixed solution (acetic acid: methanol / 1:9) and stir at room temperature for 15 minutes, then spin off the methanol, wash with aqueous sodium bicarbonate and separate through silica gel column (dichloromethane: methanol / 20:1) to obtain the target Product, yield 71%. 1 H NMR (400MHz, DMSO) δ (ppm) 7.90-7.88 (d, J = 7.48Hz, 2H), 7.82-7.80 (d, J = 7.8Hz, 1H), 7.71-7.69 (m, 2H), 7.43- 7.31(m,8H),5.21(s,2H),4.36-4.26(m,3H),4.22-4.21(m,1H),3.97-3.92(t,J=9.48Hz,1H),3.87-3.80( m,1H),3.62(s,3H),3.51-3.47(m,1H),1.86-1.82(m,2H).13C NMR(400MHz,DMSO)δ172.82,156.07,152.86,151.95,143.74,142.56,140.73 ,139.41,137.41,135.61,128.92,128.48,128.39,12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com