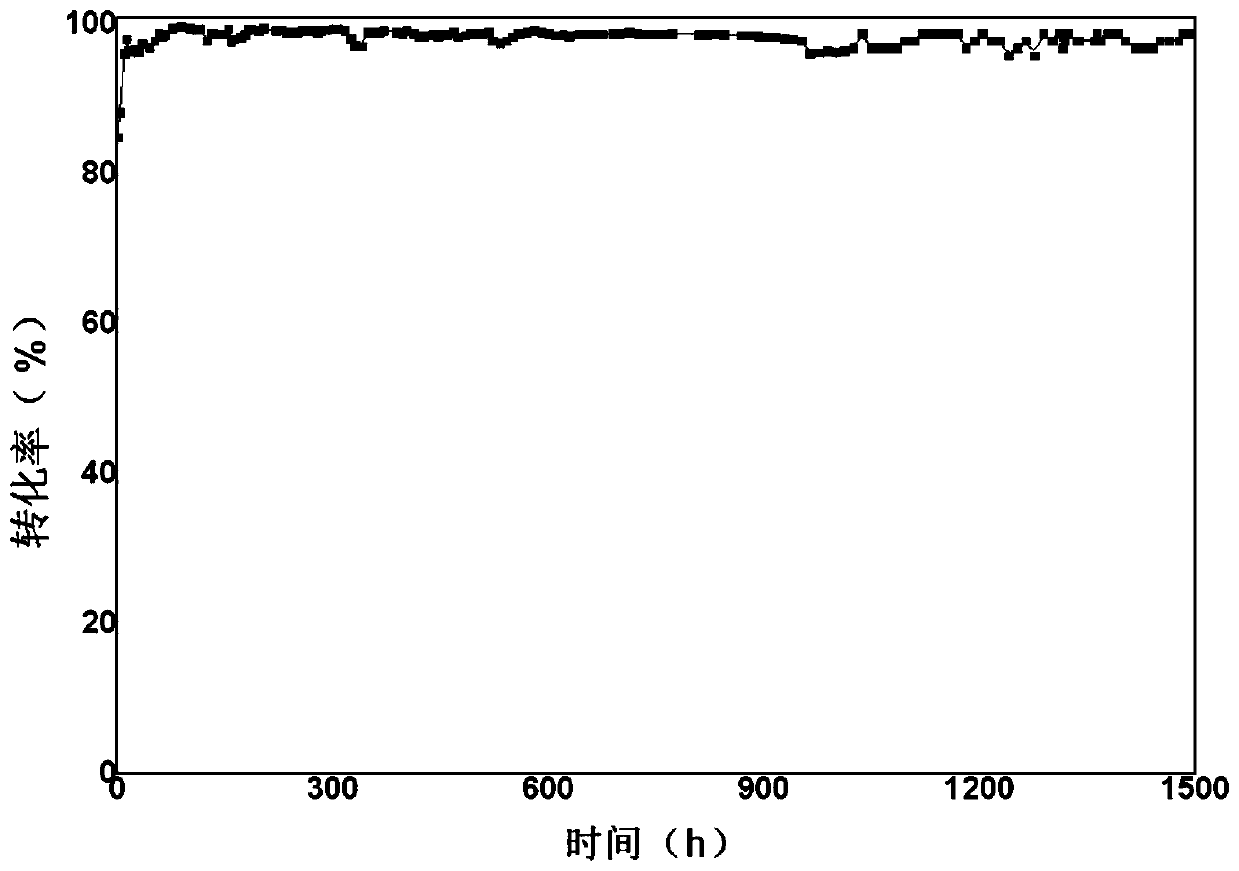
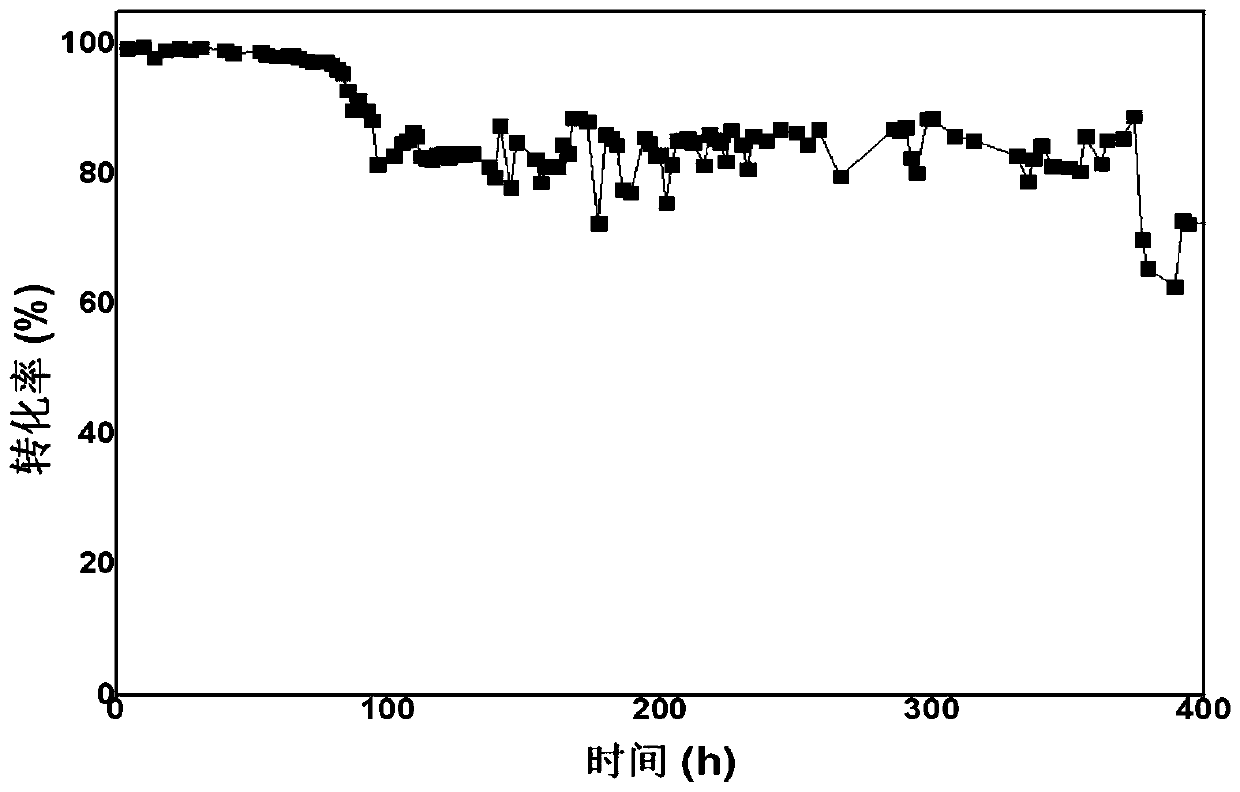
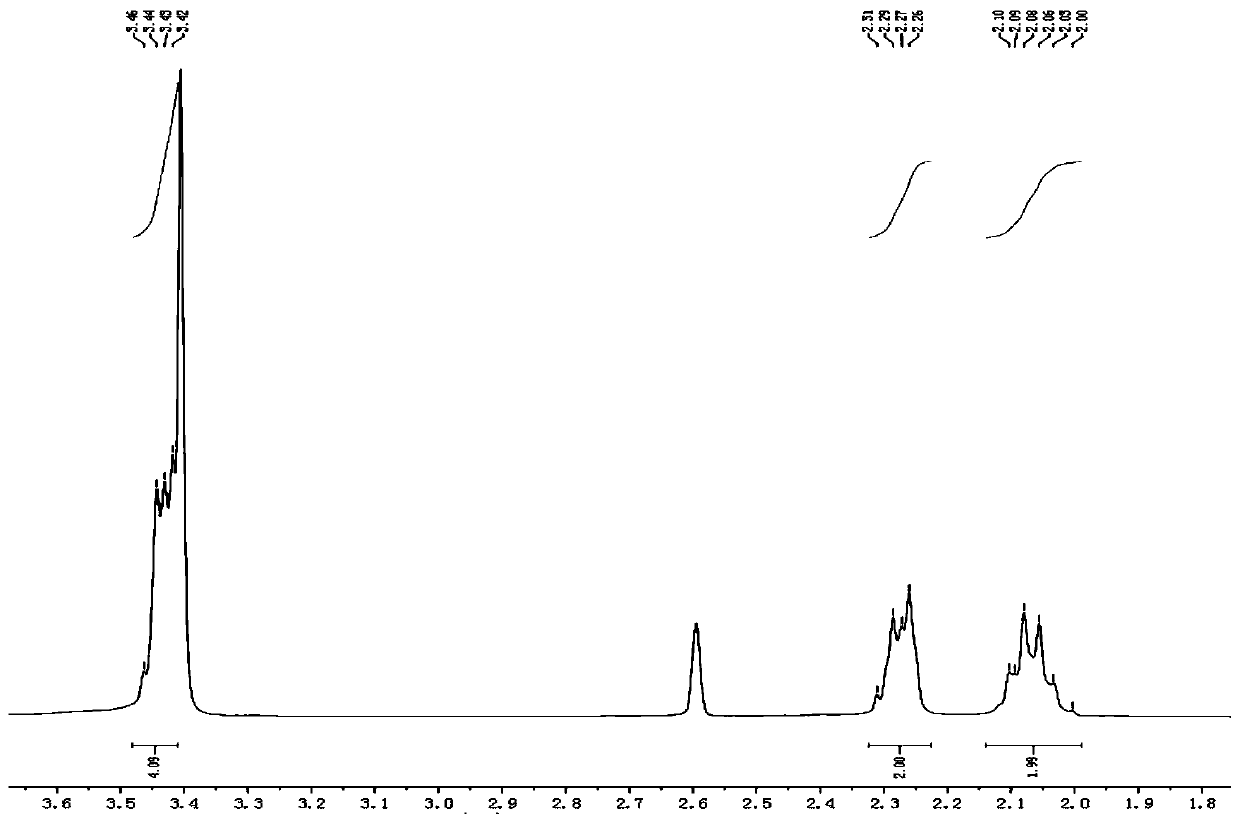
Synthetic method of 1,2,4,5-cyclohexanetetracarboxylic dianhydride
A technology of cyclohexanetetracarboxylic acid and pyromellitic acid, which is applied in directions such as organic chemistry, can solve the problems of high price of rhodium carbon, long process route, low production efficiency, etc., achieves good economy and practicability, and reduces operating procedures , the effect of improving the efficiency of esterification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Synthesis of ethyl pyromellitic acid
[0029] Add 1 kg of pyromellitic acid and 3.83 kg of triethyl orthoacetate into a 5 L three-necked flask equipped with a thermometer, mechanical stirring and a reflux condenser, and heat to 145-150 ° C in an oil bath for reflux reaction for 12 hours. After the reaction is completed, recover the remaining solvent under reduced pressure, then add 500g of absolute ethanol to the residue, cool down to 0-5°C, stir and crystallize for 1h, filter with suction, wash the filter cake with 100g of absolute ethanol, and dry under reduced pressure at room temperature to obtain white crystals 1.22 kg of ethyl pyromellitic acid, yield 85%, HPLC purity 99.6%.
[0030] (2) Preparation of hydrogenated pyromellitic acid ethyl ester
[0031] Preparation of hydrogenated pyromellitic acid ester: dissolving tetraethyl pyromellitic acid in absolute ethanol to form an ethanol solution containing 20% (wt) tetraethyl pyromellitic acid as the reaction r...
Embodiment 2
[0037] (1) Synthesis of methyl pyromellitic acid
[0038] Add 2kg of pyromellitic acid and 10kg of trimethyl orthoformate into a 10L three-necked flask equipped with a thermometer, mechanical stirring and a reflux condenser, and heat to 105-110°C in an oil bath for 24 hours. During the reaction, components with low boiling points were continuously removed. After the reaction is completed, recover the remaining solvent under reduced pressure, then add 800g of absolute ethanol to the residue and cool down to -5-0°C, stir and crystallize for 2h, filter with suction, wash the filter cake with 100g of absolute ethanol, and dry under reduced pressure at room temperature to obtain white 2.59 kg of crystal pyromellitic acid methyl ester, yield 90%, HPLC purity 99.3%.
[0039] (2) Preparation of hydrogenated pyromellitic acid methyl ester
[0040] Preparation of hydrogenated pyromellitic acid methyl ester: dissolving tetramethyl pyromellitic acid in anhydrous methanol to form a metha...
Embodiment 3
[0045] (1) Synthesis of ethyl pyromellitic acid
[0046] Add 500g of pyromellitic acid, 957g of triethyl orthoacetate, and 2L of toluene into a 5L three-necked flask equipped with a thermometer, mechanical stirring and reflux condenser, and heat to 125-140°C in an oil bath for reflux reaction for 8 hours. After the reaction is completed, recover the remaining solvent under reduced pressure, then add 300g of absolute ethanol to the residue, cool down to 0-5°C, stir and crystallize for 1h, filter with suction, wash the filter cake with 100g of absolute ethanol, and dry under reduced pressure at room temperature to obtain white crystals 504.5 g of ethyl pyromellitic acid, yield 70%, HPLC purity 99.1%.
[0047] (2) Preparation of hydrogenated pyromellitic acid ethyl ester
[0048] Preparation of hydrogenated pyromellitic acid ethyl ester: dissolving tetraethyl pyromellitic acid in absolute ethanol to form an ethanol solution containing 20% (wt) tetraethyl pyromellitic acid as t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com