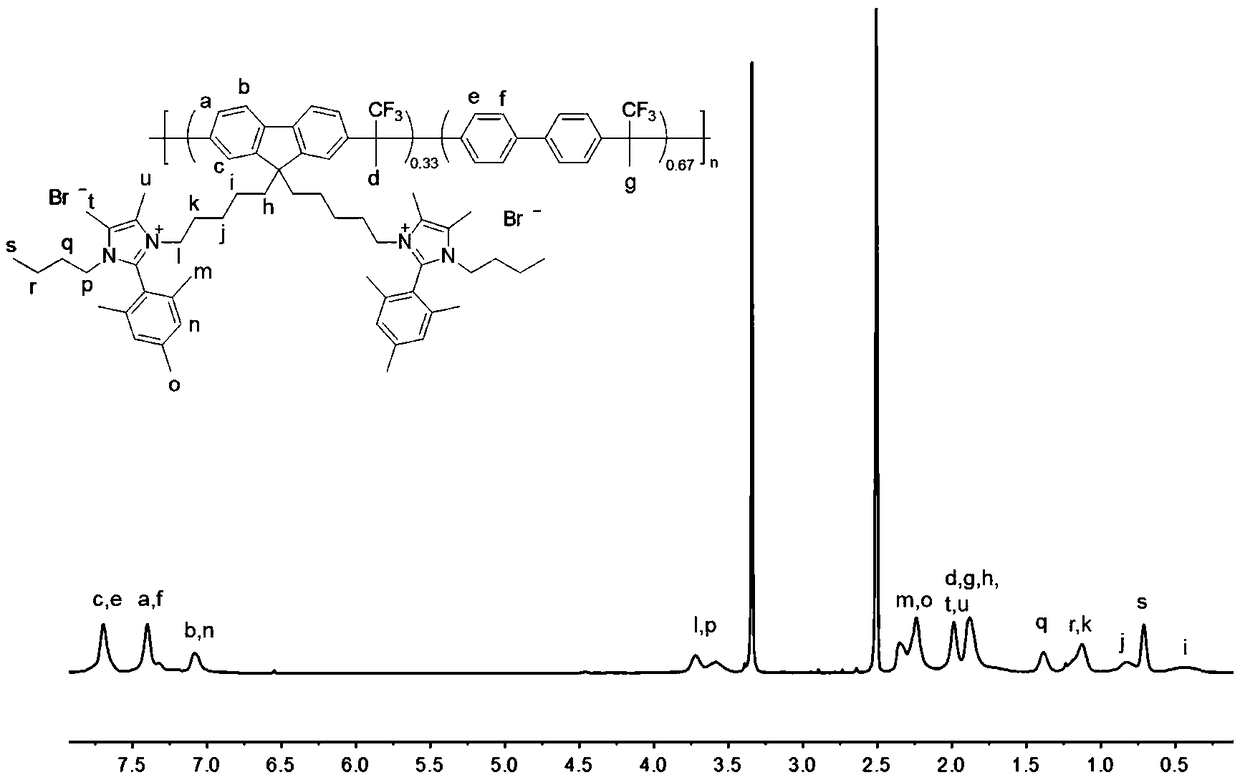
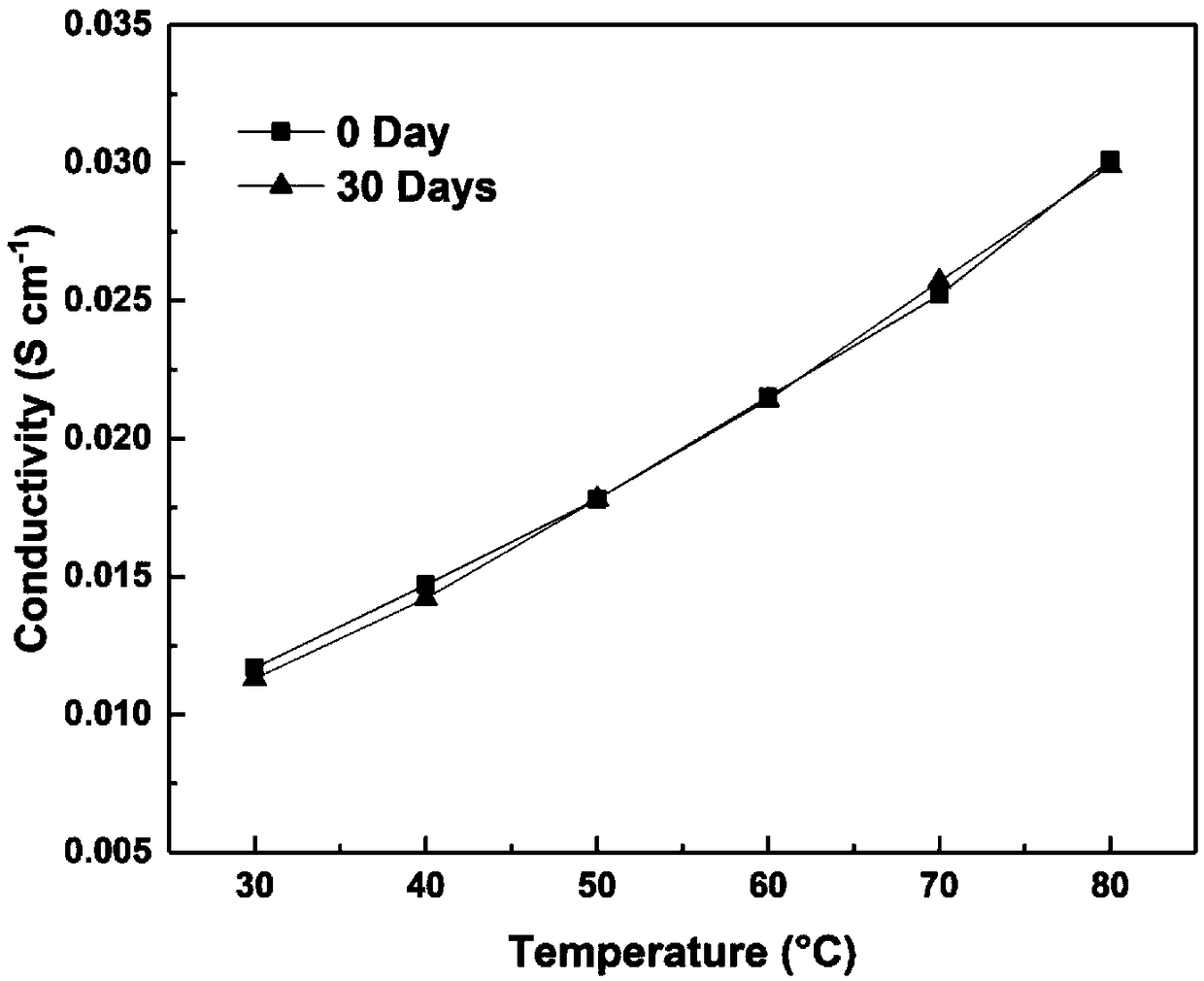
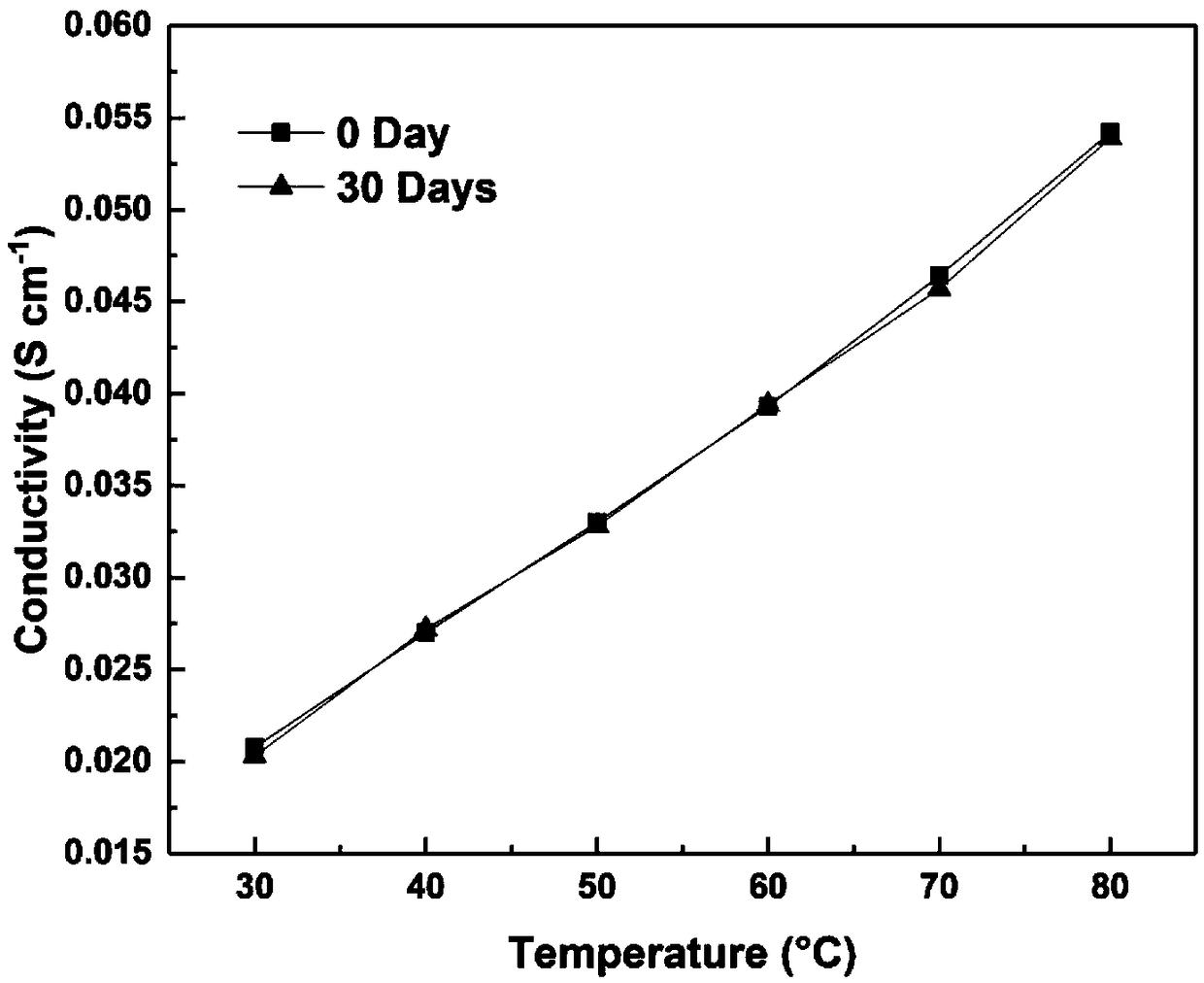
Cation group-containing ether bond-free polyfluorene alkylene, preparation method thereof and anion exchange membrane
A technology of cationic groups and polyfluoranes, which is applied in the field of anion exchange membranes, can solve the problems of decreased mechanical properties of anion exchange membranes, decreased performance of anion exchange membranes, excessive swelling of membranes, etc., and achieves the effect of excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention provides a preparation method of cationic group-containing polyfluorene alkylene without ether bond with structure of formula (I), comprising:
[0056] A) dissolving fluorene-type monomers, comonomers, and ketone monomers of formula (II) in a solvent, and reacting under the condition of superacid as a catalyst, the polymer obtained; the comonomers are selected from the group consisting of One or more of benzene, p-terphenyl, m-terphenyl, naphthalene and anthracene;
[0057] B) dissolving the polymer in an aprotic polar solvent and reacting with one of tertiary amine, imidazole and tertiary phosphine to obtain a cationic group-containing polyfluorene alkylene without ether linkage of the structure of formula (I);
[0058]
[0059] Wherein, Y is one of F, Cl, Br and I; a is 0-11.
[0060] The preparation method of the cationic group-containing non-ether bond polyfluorene alkylene with the structure of formula (I) provided by the present invention...
Embodiment 1
[0104] (1) In a 75mL pressure-resistant bottle with a magnetic stirrer, dissolve 2.32g of a=4 fluorene monomer, 1.54g of biphenyl, and 1.68g of 1,1,1-trifluoroacetone in 11mL of dichloromethane, Under ice-water bath, add 11mL CF 3 SO 3 H. After capping for 6 hours, the system was settled in methanol to obtain a white solid, which was washed several times with methanol and dried in vacuo. The yield is 97%, and the intrinsic viscosity is 0.5dL / g (determined in NMP at 25°C).
[0105] (2) In a 100mL single-necked flask equipped with a magnetic stir bar, add 1g of the obtained white solid and 0.77g image 3 Add 20mL of N,N-dimethylacetamide to dissolve the large sterically hindered imidazole, react at 80°C for 72h, cool to room temperature and precipitate ionized polymer in ether, wash with ether several times, and vacuum dry for 12h to obtain The imidazolium salt group has no ether linkage polyfluorenalkylene. The yield is 95%, and the intrinsic viscosity is 0.8dL / g (determine...
Embodiment 2
[0109] (1) In a 75mL pressure-resistant bottle with a magnetic stirrer, dissolve 1.48g of a=5 fluorene-type monomer and 1.61g of terphenyl, 1.12g of 1,1,1-trifluoroacetone in 15mL of dichloromethane, Under ice water bath, add 10mL SbF 5 After capping and reacting for 3 h, the system was settled in methanol to obtain a white solid, which was washed several times with methanol and dried in vacuo. The yield is 98%, and the intrinsic viscosity is 0.6dL / g (determined in NMP at 25°C).
[0110] (2) In a 75mL pressure-resistant bottle equipped with a magnetic stirrer, add 1g of the obtained white solid and 0.60g of trimethylamine in water, add 20mL of dimethyl sulfoxide to dissolve, react at room temperature for 48h, and then precipitate and ionize in ether After the polymer was washed several times with ether, it was vacuum-dried for 12 hours to obtain a polyfluorene alkylene containing quaternary ammonium cationic groups without ether linkages. The yield is 94%, and the intrinsic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com