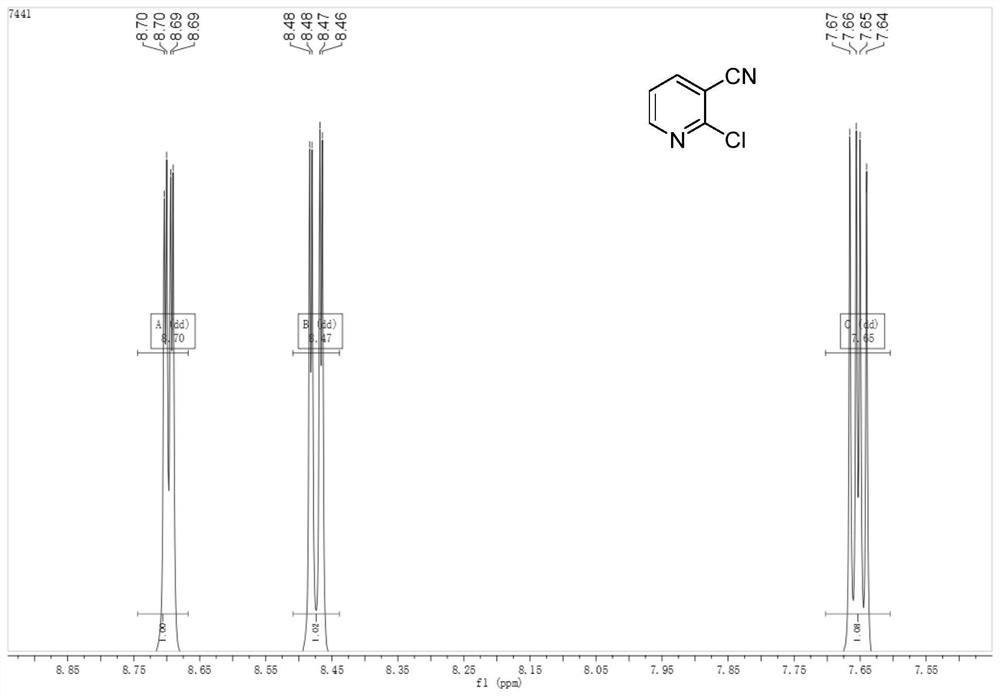
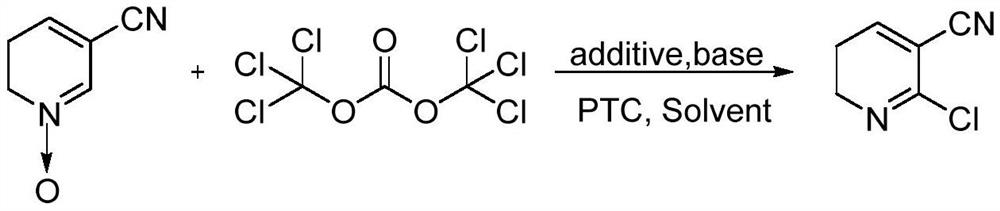
A kind of green preparation method of 2-chloro-3-cyanopyridine
A cyanopyridine and green technology, which is applied in the field of green preparation of 2-chloro-3-cyanopyridine, can solve problems such as difficulty in treating phosphorus-containing wastewater and waste acid, achieves low environmental impact, high purity and yield, simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take a dry four-neck flask, set up a tail gas absorption device, add 15g (125mmol) 3-cyanopyridine N-oxide and 110mL 1,2-dichloroethane, stir at a low temperature of 0°C to form a homogeneous solution , when T=-5~0℃, add 25.30g (250mmol) triethylamine, continue cooling, add 3.50g (62.5mmol) sodium chloride, 0.78g (6.25mmol) sodium sulfite, 1.13g (5mmol) benzyl Triethylammonium chloride, continued cooling to -15°C, began to slowly add 60mL of 1,2-dichloroethane solution containing 18.52g (62.5mmol) bis(trichloromethyl)carbonate dropwise, and the dropwise addition was completed. The reaction was incubated at this temperature for 16 hours. After the reaction was completed, 40 mL of deionized water was added dropwise and stirred to quench. Separate the layers, wash the organic phase with 60 mL of 10% sodium hydroxide solution, then wash with 60 mL of deionized water, and finally extract the aqueous phase with (60 mL*2) 1,2-dichloroethane, combine the organic layers, add act...
Embodiment 2
[0024] Take a dry four-neck flask, set up a tail gas absorption device, add 15g (125mmol) 3-cyanopyridine N-oxide and 110mL 1,2-dichloroethane, stir at a low temperature of 0°C to form a homogeneous solution , when T=-5~0℃, add 31.63g (312.5mmol) triethylamine, continue cooling, add 1.83g (31.25mmol) sodium chloride, 0.78g (6.25mmol) sodium sulfite, 1.13g (5mmol) benzyl Triethylammonium chloride, continue to cool to -15°C, start to slowly add 60mL of 1,2-dichloroethane solution containing 18.52g (62.5mmol) bis(trichloromethyl)carbonate dropwise, dropwise , keep the reaction at this temperature for 16 hours, after the reaction is complete, add 40 mL of deionized water dropwise and stir to quench. Separate the layers, wash the organic phase with 60 mL of 10% sodium hydroxide solution, then wash with 60 mL of deionized water, and finally extract the aqueous phase with (60 mL*2) 1,2-dichloroethane, combine the organic layers, add activated carbon at 60 Reflux and decolorize at ℃ ...
Embodiment 3
[0026] Take a dry four-neck flask, set up a tail gas absorption device, add 15g (125mmol) 3-cyanopyridine N-oxide and 110mL 1,2-dichloroethane, stir at a low temperature of 0°C to form a homogeneous solution , when T=-5~0 ℃, add 31.63g (312.5mmol) triethylamine, continue cooling, add 3.50g (62.5mmol) sodium chloride, 0.78g (6.25mmol) sodium sulfite, 1.13g (5mmol) benzyl Triethylammonium chloride, continue to cool to -15°C, start to slowly add 60mL of 1,2-dichloroethane solution containing 18.52g (62.5mmol) bis(trichloromethyl)carbonate dropwise, dropwise , keep the reaction at this temperature for 15 hours, after the reaction is complete, add 40 mL of deionized water dropwise and stir to quench. Separate the layers, wash the organic phase with 60 mL of 10% sodium hydroxide solution, then wash with 60 mL of deionized water, and finally extract the aqueous phase with (60 mL*2) 1,2-dichloroethane, combine the organic layers, add activated carbon, and Reflux and decolorize at 60°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com