A kind of pharmaceutical compound and its preparation and application
A technology of compounds and uses, applied in the field of pharmaceutical compounds and their preparation and uses, can solve the problems of hydrolysis, absorption, lowering of TXA2/PGI2 ratio, gastrointestinal mucosal damage and other problems in the real sense, so as to avoid gastrointestinal bleeding , reduce the resistance effect, the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
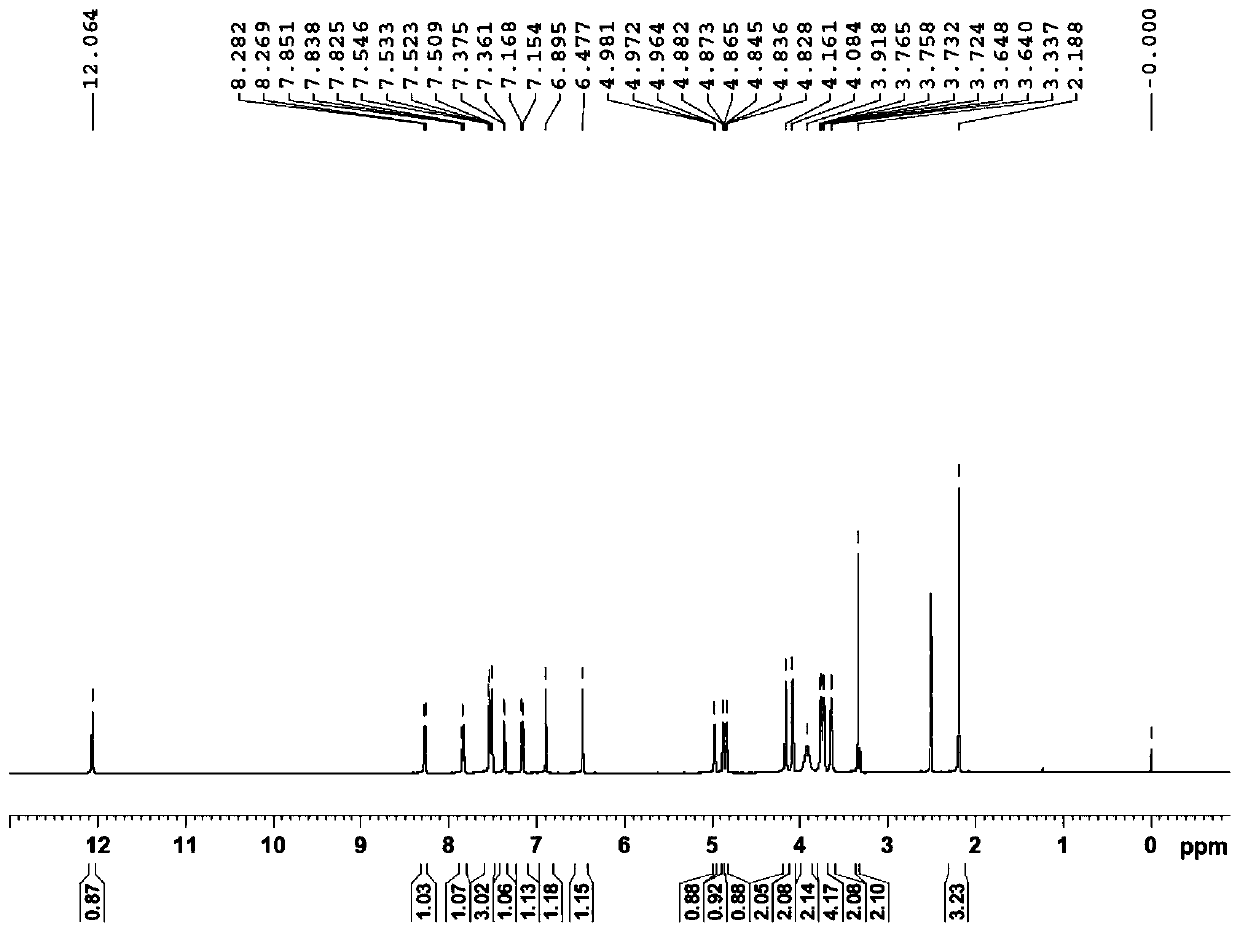
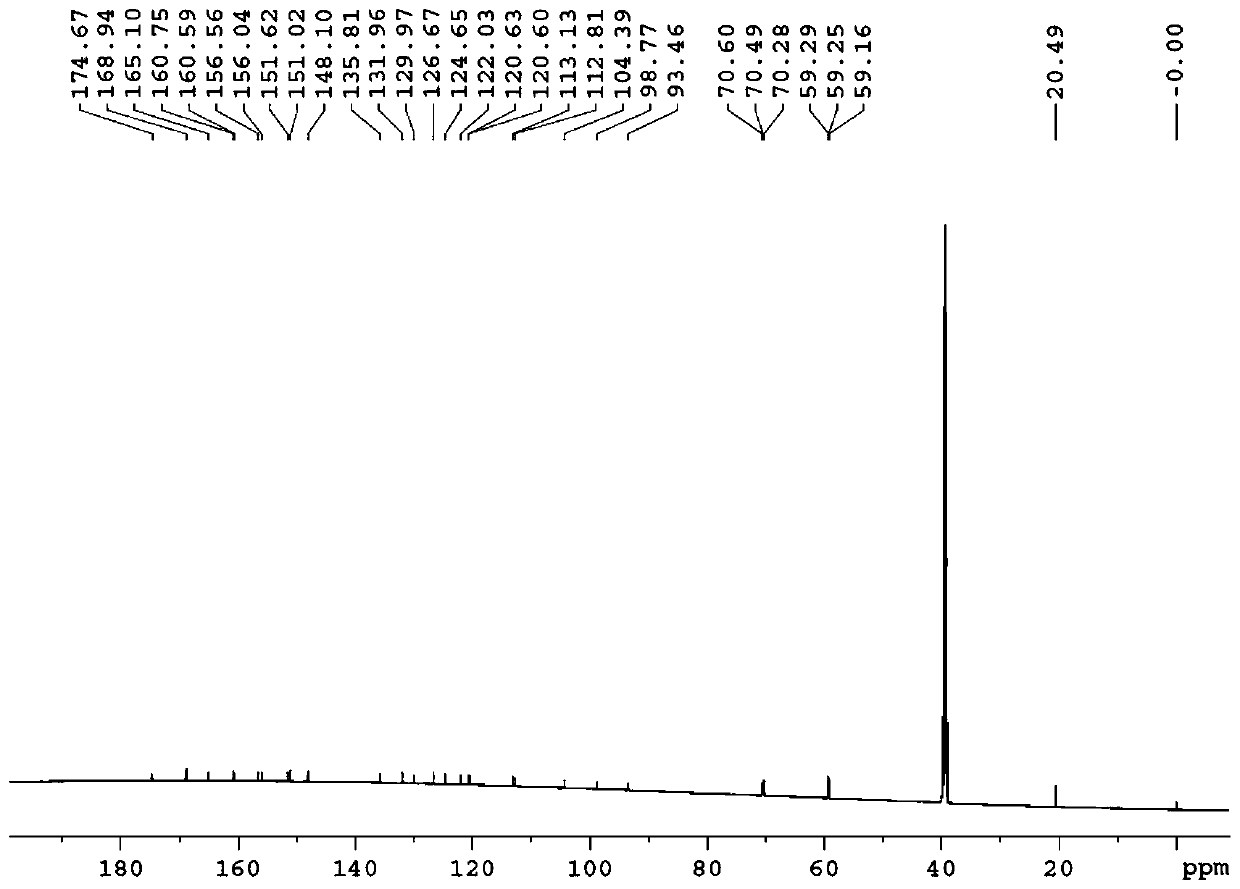
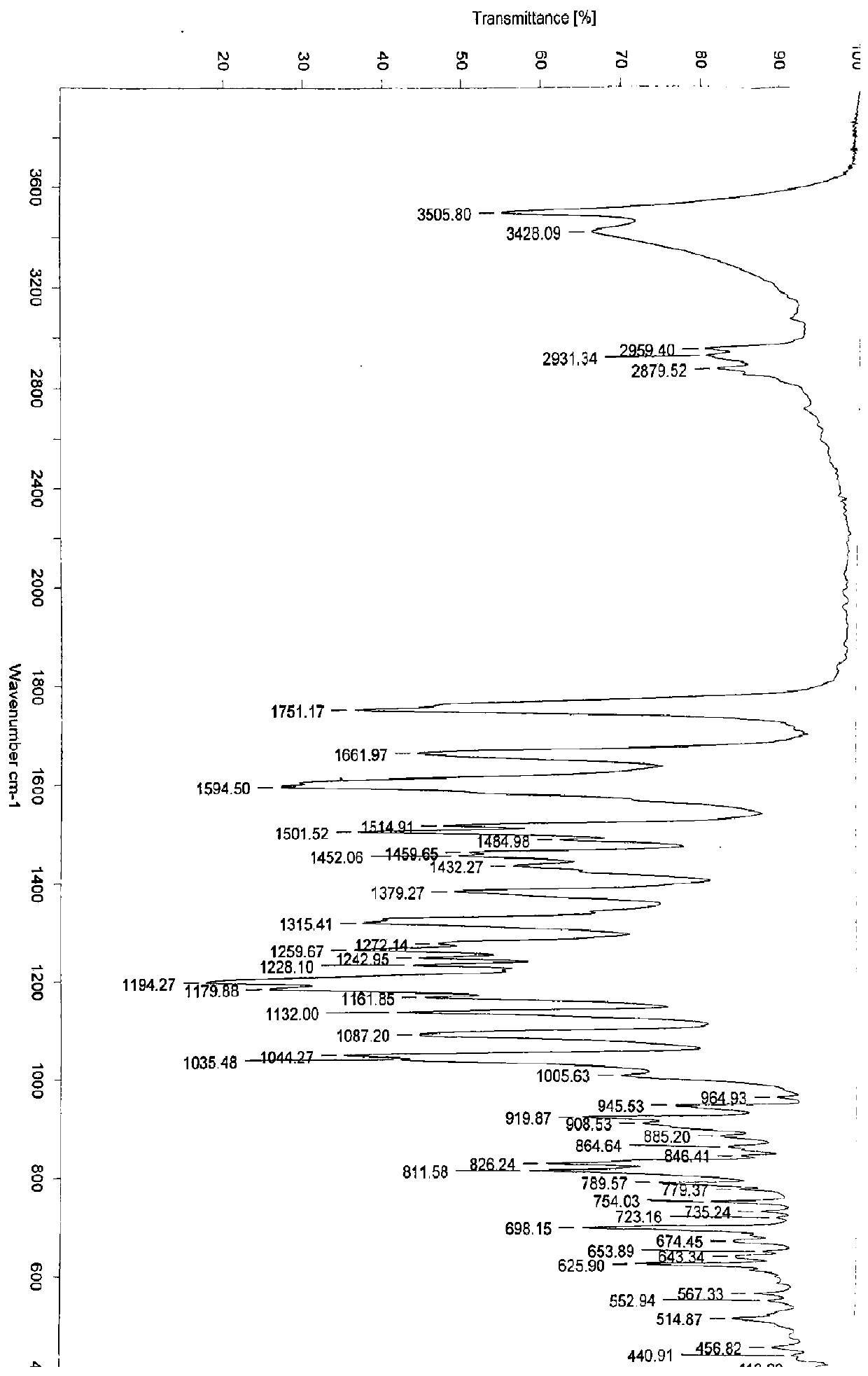
[0154] Example 1: Preparation of 3-O-((2-acetoxy)-benzoyl)-3',4',7-tri(-O-hydroxyethyl)quercetin
[0155] 1) Troxerutin (10g=10.9mmol, troxerutin chromatographic purity 85%, content 81%) was dissolved in 380ml of water, then added 20ml of concentrated sulfuric acid, heated to reflux for 3 hours, stopped the reaction and cooled down, and cooled to After filtering at room temperature, and then washing with water until neutral, the filter cake was obtained, and vacuum-dried at 65°C to obtain 5.66 g of 3′,4′,7-tris(-O-hydroxyethyl)quercetin with a chromatographic purity of 90.2%. Yield 56.6%.
[0156] 2) In a 100ml three-necked reaction flask, anhydrous 3′,4′,7-tris(-O-hydroxyethyl)quercetin (2g=4.6mmol, water content less than 0.1%) was stirred and dissolved at room temperature in In DMF (40ml), add aspirin (1.66g=9.2mmol) again, then add DCC (1.9g=9.2mmol) and 10% DMAP therein, stir reaction at -5~5 ℃, after 12 hours, reaction finishes, The reaction solution was filtered, and ...
Embodiment 2
[0159] Example 2: Preparation of 3-O-((2-acetoxy)-benzoyl)-3',4',7-tri(-O-hydroxyethyl)quercetin
[0160] (1) Troxerutin (100g=119.9mmol, troxerutin chromatographic purity 92%, content 89%) was dissolved in 3800ml water, then added 200ml concentrated sulfuric acid, heated to reflux for 3 hours, stopped the reaction, and kept warm for 90 Filtrate above ℃, wash with hot water until neutral to obtain a filter cake, and dry it in vacuum at 65℃ to obtain 55.4g of 3′,4′,7-tris(-O-hydroxyethyl)quercetin with a chromatographic purity of 93.8%. Yield 55.4%.
[0161] (2) In a 500ml glass reaction vial, dissolve 10g (23.0mmol) of 3′,4′,7-tris(-O-hydroxyethyl)quercetin obtained in (1) in DMF (200ml) for later use, Add 12.4g (68.9mmol) of aspirin, add 12g of DCC and 12% DMAP to it, stir the reaction at -5~-15°C, after 18 hours, the reaction is completed, the reaction solution is filtered, and the filtrate is added with 15 times saturated ice salt water, stirred, and precipitated The soli...
Embodiment 3
[0163] Example 3: Preparation of 3-O-((2-acetoxy)-benzoyl)-3',4',7-tri(-O-hydroxyethyl)quercetin
[0164] (1) Troxerutin (100g=121.3mmol, troxerutin chromatographic purity 93%, content 90%) was dissolved in 3800ml water, then added 200ml concentrated sulfuric acid, heated to reflux for 3 hours, stopped the reaction, and kept warm for 80 Filtrate above ℃, then wash with hot water until neutral to obtain a filter cake, add 4500ml of ethanol-water (40:60) mixed solvent to the filter cake, stir and heat to reflux for 30 minutes, heat filter, and vacuum-dry the filter cake at 65 °C to obtain 3' , 4', 7-tris(-O-hydroxyethyl) quercetin 53.1g, chromatographic purity 95.1%, yield 53.1%.
[0165] (2) In a 500ml glass reaction vial, dissolve 10g (23.0mmol) of 3′,4′,7-tris(-O-hydroxyethyl)quercetin obtained in (1) in DMF (200ml) for later use, Add 8.3g (46.1mmol) of aspirin, then add 15g of DCC and 15% DMAP to it, and stir the reaction at -15~-25°C. After 26 hours, the reaction is comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com