Drag sustained release stent
A drug and slow-release technology, applied in stents, medical science, coatings, etc., can solve the problems of time asynchronous matching that affect patients' breathing, stent displacement, and mucosal outcome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
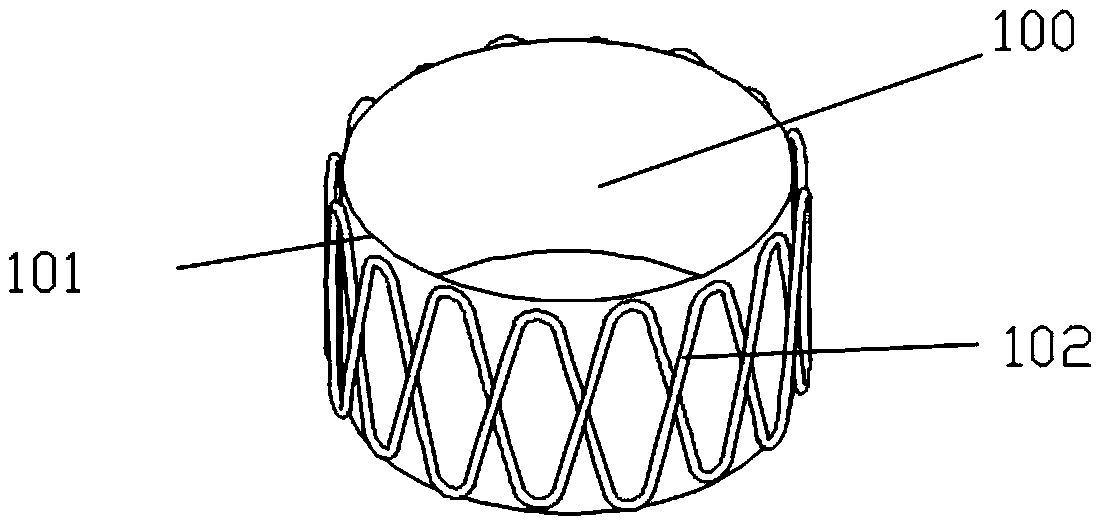
[0074] Such as Figure 2-Figure 3 As shown, a stent graft 100 includes a stent 102 and a polymer film 101, and the stent 102 and the polymer film 101 are connected together to form a stent graft. The polymer film is a degradable polymer film.
[0075] The stent 102 of the present invention consists of a single degradable fiber thread that is twisted and wound on a weaving mold to form a network-like hollow structure. The ester ratio is 10:90 or 15:85, and the intrinsic viscosity is 1.0-1.6dl / g). The stent body composed of the degradable stent wire will be completely degraded within 30-90 days after being implanted in the nasal cavity.
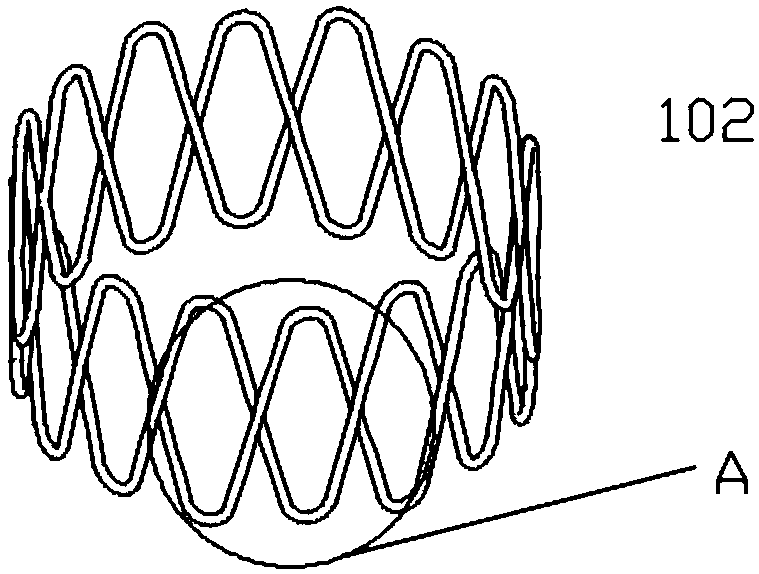
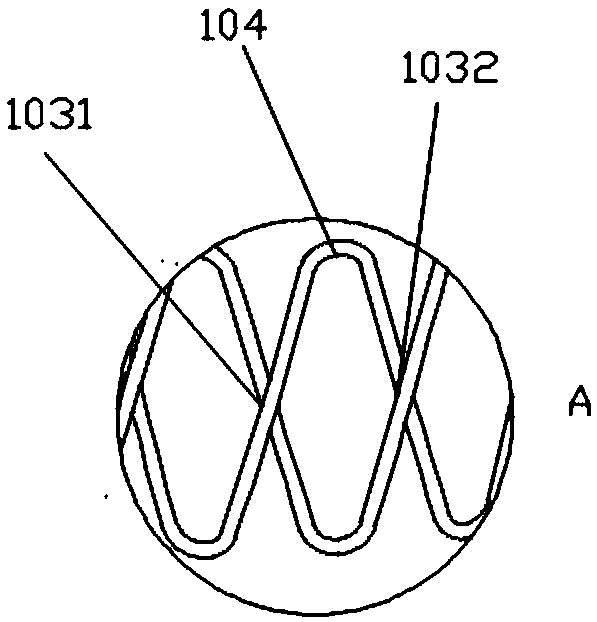
[0076] The upper and lower edges of the bracket include 15 corolla 104 (apex), and the silk threads are interlaced and superimposed together to form intersections 103. An intersection point 1031 is located above the reversely wound silk thread, and then the next immediately adjacent intersection point 1032 is located below the reversely wound...
Embodiment 2
[0082] A stent graft 100, similar to Embodiment 1, the difference is that the polymer film 101 used in this embodiment is a straight cylindrical non-degradable polymer film made of polyvinyl chloride, or polyurethane, polyamide and other materials , the thickness of the film is about 0.1-0.5mm, and its diameter is the same as figure 1 The illustrated stents 102 have comparable inner diameters. Such as Figure 6 As shown, there is a traction line 106 formed by crossing non-degradable sutures (polypropylene or nylon, etc.) in the middle of the polymer film, and the traction line 106 can be clamped by the tool and pulled out of the nasal cavity to bring the polymer film out of the nasal cavity .
[0083] Such as figure 1 As shown, the polymer film 101 and the stent 102 are sutured together at the corolla 104 to form a covered stent 100 through a degradable suture (such as Jiaxiu pure natural collagen suture from Hunan Ranyuan Company), and the polymer film 101 is sutured on O...
Embodiment 3
[0085] This embodiment is a drug sustained-release stent loaded with a drug coating, which may include the stent graft 100 shown in Embodiment 1 or Embodiment 2. The stent graft 100 includes a stent 102 and a polymer film 101, a stent 102 and a polymer membrane. The material membranes 101 are connected together to form a stent graft. Both the stent 102 and the polymer film are provided with a layer of drug coating, and the drug coating on the stent 102 and the polymer film 101 is composed of controlled-release polymers and drugs such as anti-inflammatory drugs, antibacterial drugs, anticancer drugs or hemostatic drugs. mixed.
[0086] Specifically, the stent graft 100 of this embodiment is the second stent graft 100 (degradable polymer film) in the first embodiment, and the drug coating on the polymer film 101 is released faster than the drug coating on the stent 102. . The drug coating on the polymer film 101 may have multiple variations, for example, the same drug as that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com