Preparation method of 5-bromolevulinic acid
A kind of technology of bromolevulinic acid and trifluoroacetic acid, applied in the field of preparation of 5-bromolevulinic acid, can solve the problem of not finding the preparation of 5-bromolevulinic acid, etc., and achieve the effect of high raw material utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
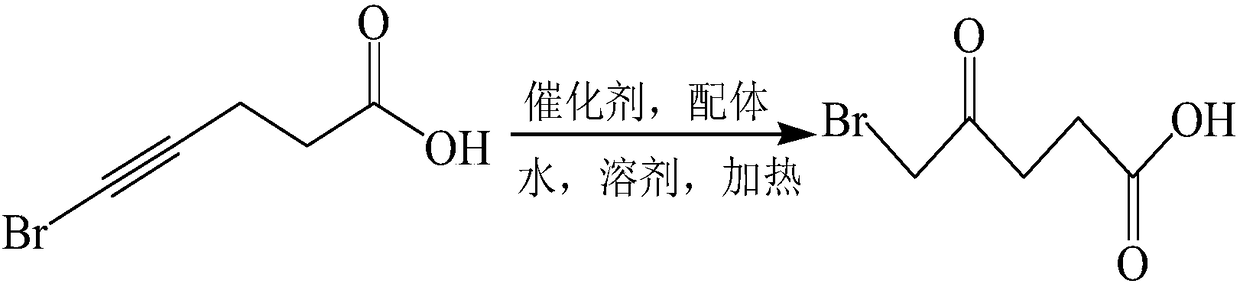
[0026] A preparation method of 5-bromolevulinic acid, comprising the steps of: dissolving 5-bromo-4-pentynoic acid in a solvent, then adding water, copper (II) salt and ligand at 25-90°C After reacting for 4-6 hours, 5-bromolevulinic acid is isolated; wherein, the ligand is at least one selected from 1,10-phenanthroline or its derivatives.
[0027] The preparation method of 5-bromolevulinic acid provided by the embodiments of the present invention is to dissolve 5-bromo-4-pentynoic acid in a solvent as a raw material, and after adding water, add copper (II) salt and 1,10-phenanthrene Roline or derivatives thereof are heated and reacted as a ligand, thereby preparing 5-bromolevulinic acid, and its specific reaction equation is as follows:
[0028]
[0029] The preparation method of 5-bromolevulinic acid provided by the embodiment of the present invention can obtain 5-bromolevulinic acid with high selectivity, and the yield is high, and the method for preparing 5-bromolevulin...
Embodiment 1
[0039] The embodiment of the present invention provides a kind of 5-bromolevulinic acid, and its specific preparation method comprises the following steps:
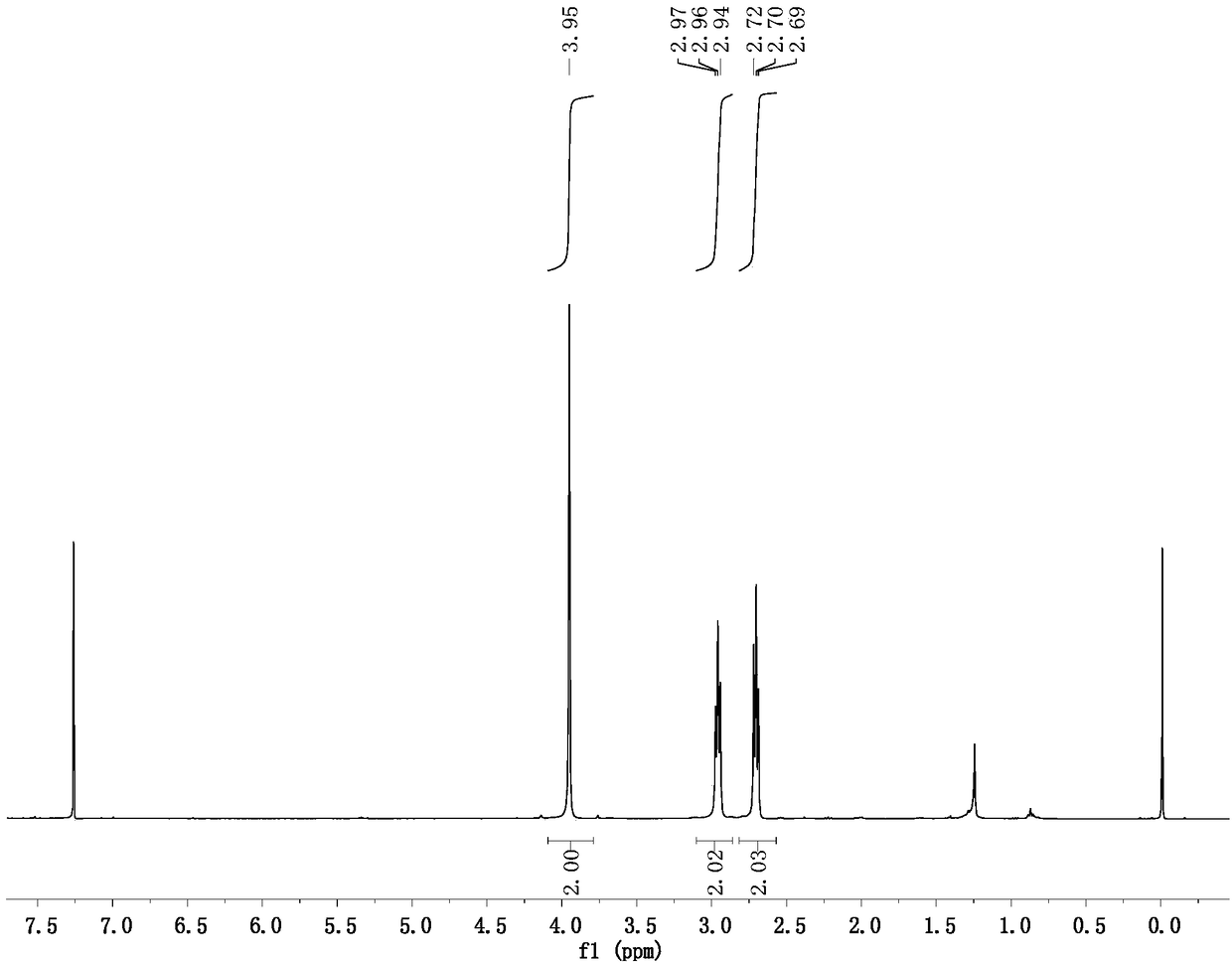
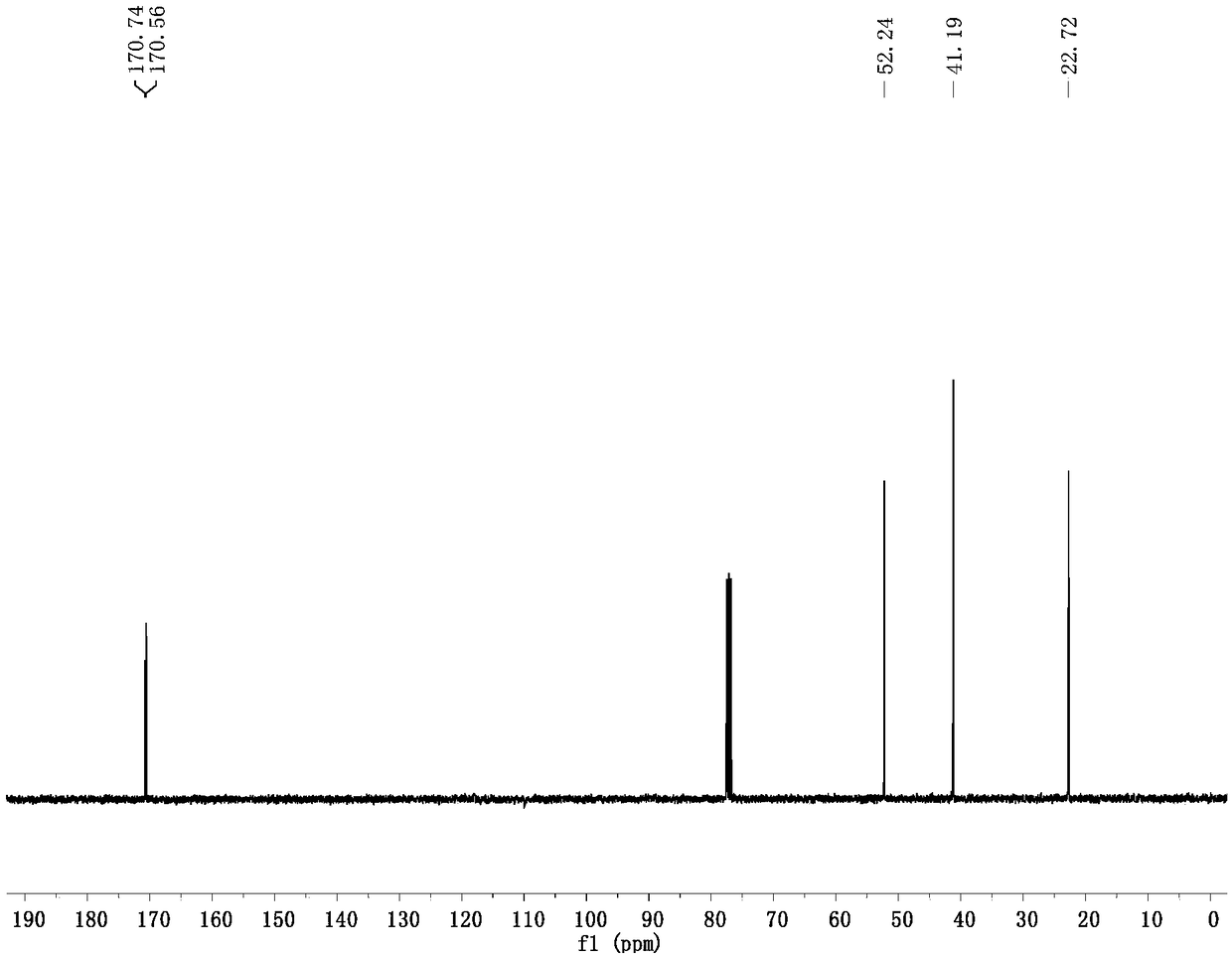
[0040] Prepare a 100mL flask, dissolve 0.531g of 5-bromo-4-pentynoic acid (3.0mmol) into 36mL of TFA, stir well and add 0.162mL of water (9.0mmol), 0.048g of anhydrous copper sulfate (0.30mmol ), heated to 70°C for 4h, evaporated the solvent under reduced pressure, and separated by column chromatography (using ethyl acetate / petroleum ether=1:3 eluent to wash the adsorption column) to obtain 0.374g of 5-bromolevulinic acid , the yield was 64%.
Embodiment 2
[0042] Embodiment 2 is similar to the implementation steps of Example 1, but the catalyst anhydrous copper sulfate is changed to 0.040g anhydrous copper chloride (0.30mmol), and other steps are the same as in Example 1 to obtain 5-bromolevulinic acid 0.404g , the yield was 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com