Oxalobacter formigenes and application thereof
A technology for oxalic acid bacteria and formic acid production, applied in the field of microorganisms, can solve the problem of low ability to degrade oxalic acid, and achieve the effects of reducing calcium oxalate crystallization and increasing biomass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The screening of embodiment 1 producing formic acid oxalobacillus
[0031] 1. Material preparation
[0032] (1) Bacterial source
[0033] Bacterial source: Feces of healthy Chinese children (without infection history for three months).
[0034] (2) culture medium
[0035] Medium 1: potassium dihydrogen phosphate 0.25g / L, dipotassium hydrogen phosphate 0.25g / L, sodium acetate 0.9g / L, ammonium sulfate 0.8g / L, sodium oxalate 1g / L, yeast powder 5g / L and chloride Calcium 1g / L.
[0036] Medium 2: potassium dihydrogen phosphate 0.25g / L, dipotassium hydrogen phosphate 0.25g / L, sodium acetate 0.9g / L, ammonium sulfate 0.8g / L, sodium oxalate 1g / L and yeast powder 5g / L.
[0037] Medium 1 and 2 were both sterilized at 115° C. for 20 minutes; 2% (mass ratio) agar was added when using the above medium to prepare a solid plate (medium 1).
[0038] (3) Experimental equipment
[0039] Table 1 Summary of Experimental Instruments
[0040]
[0041]
[0042] 2. Isolation and scree...
Embodiment 2
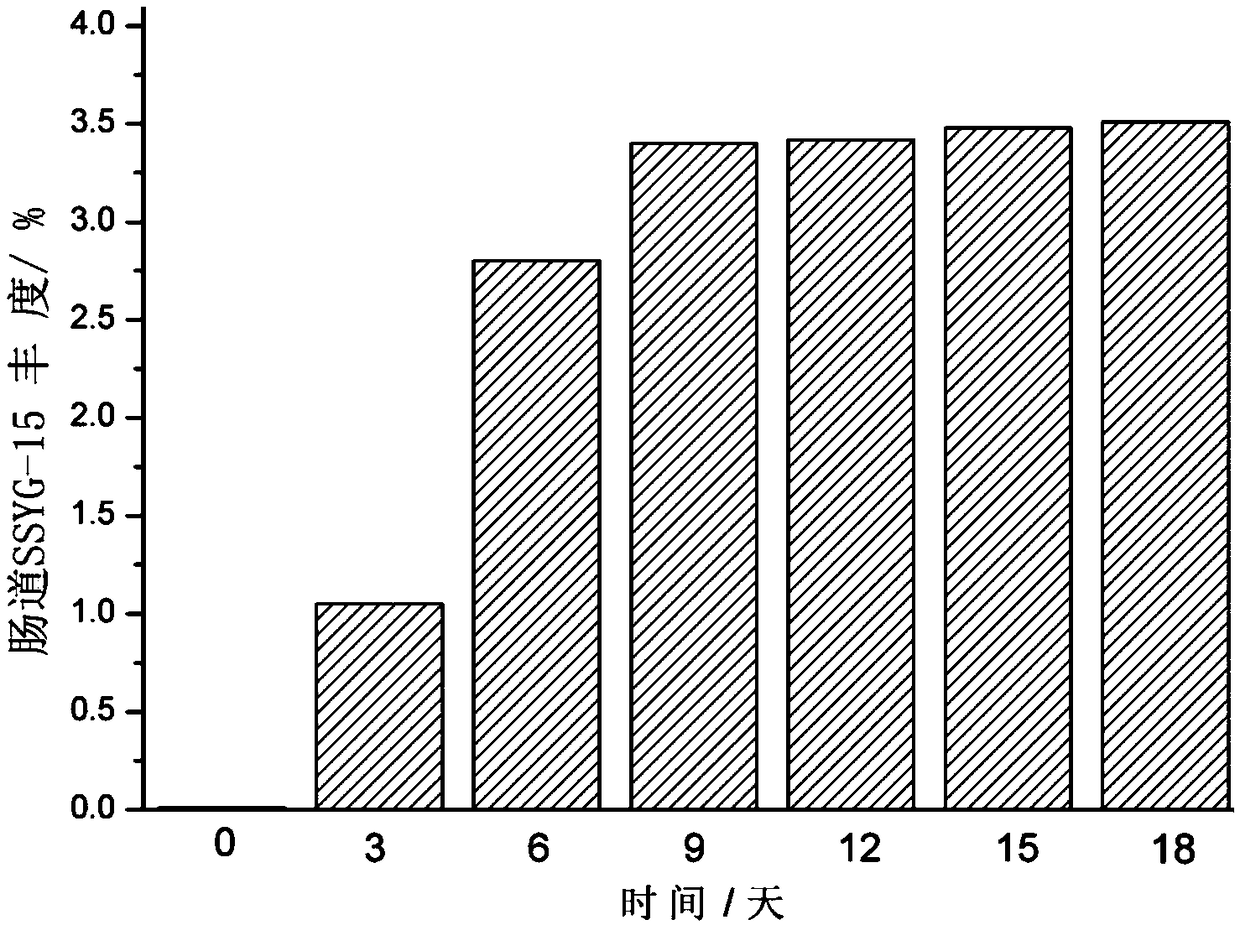
[0099] Example 2 Fermentative preparation of oxalobacterium formigenes (Oxalobacter formigenes) SSYG-15
[0100] Prepare oxalobacter formigenes (Oxalobacter formigenes) SSYG-15 according to the following steps:
[0101] (1) Prepare 250L of fermentation medium in a 500L fermenter, and steam sterilize at 115°C for 20min;
[0102] (2) During the sterilization and cooling process, feed protective gas (carbon dioxide, nitrogen, hydrogen, argon or helium) at a flow rate of 25 L / h until the fermentation broth is cooled to room temperature, and adjust the flow rate to 10 L / h;
[0103] (3) inoculate the seed liquid in the fermenter by 1%, set the tank temperature to be 37°C, and stir at a speed of 100rpm;
[0104] (4) sampling and measuring the thalline content in its fermented liquid every 12h;
[0105] (5) After 72 hours of fermentation, the cell concentration reached 2.1×10 8 CFU / mL;
[0106] (6) Take the above fermented liquid and carry out centrifugation at 4°C to obtain the b...
Embodiment 4
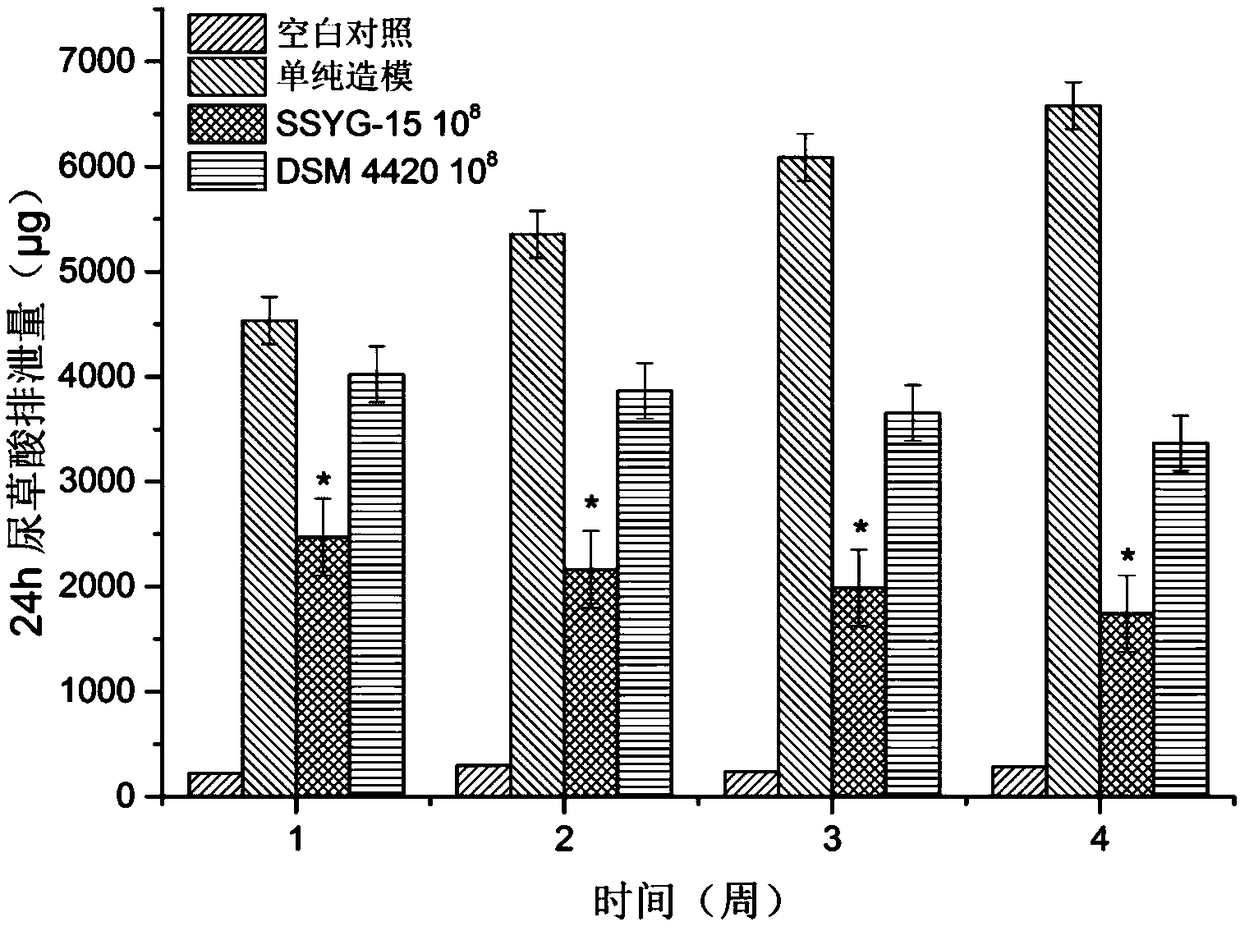
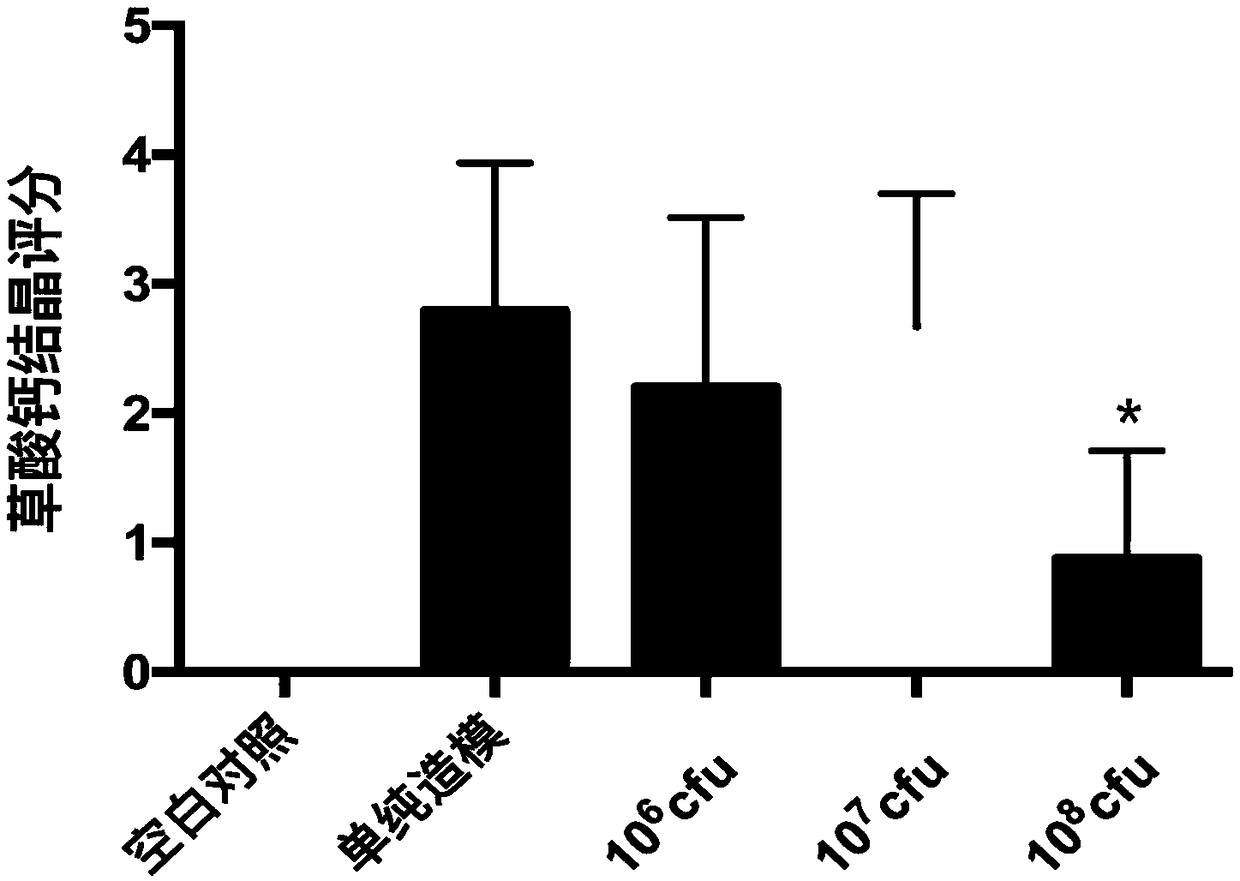
[0114] Example 4 Use of Oxalobacter formigenes SSYG-15 to reduce urinary oxalate excretion and kidney calcium oxalate crystal formation in rats with calcium oxalate stones
[0115] (1) Animals: male Sprague Dawley rats weighing 180-200g;
[0116] (2) Food: conventional feed;
[0117] (3) Administration method: gavage;
[0118] (4) Test plan:
[0119] Blank control group (n=7): fed with sterile pure water and free to drink.
[0120] Calcium oxalate stone modeling group (n=7): feeding medium and sterile pure water + 0.8% ethylene glycol, free drinking water, rats were given PBS as a placebo at 5 pm every day, and free drinking water.
[0121] SSYG-15 10 8 cfu treatment group (n=7): feeding SSYG-15 fermentation liquid (10 7 cfu / ml) 1ml and sterile pure water + 0.8% ethylene glycol, the rats were gavaged at 5:00 p.m. every day and had free access to water.
[0122] DSM 4420 10 8 cfu treatment group (n=7): feeding DSM 4420 fermented liquid (10 8 cfu / ml) 1ml and sterile pure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com