Enveloped virus resistant to complement inactivation for the treatment of cancer
A technology for transmembrane domains and proteins, applied in the field of enveloped viruses that are resistant to complement inactivation for the treatment of cancer, can solve problems such as NDV particle destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
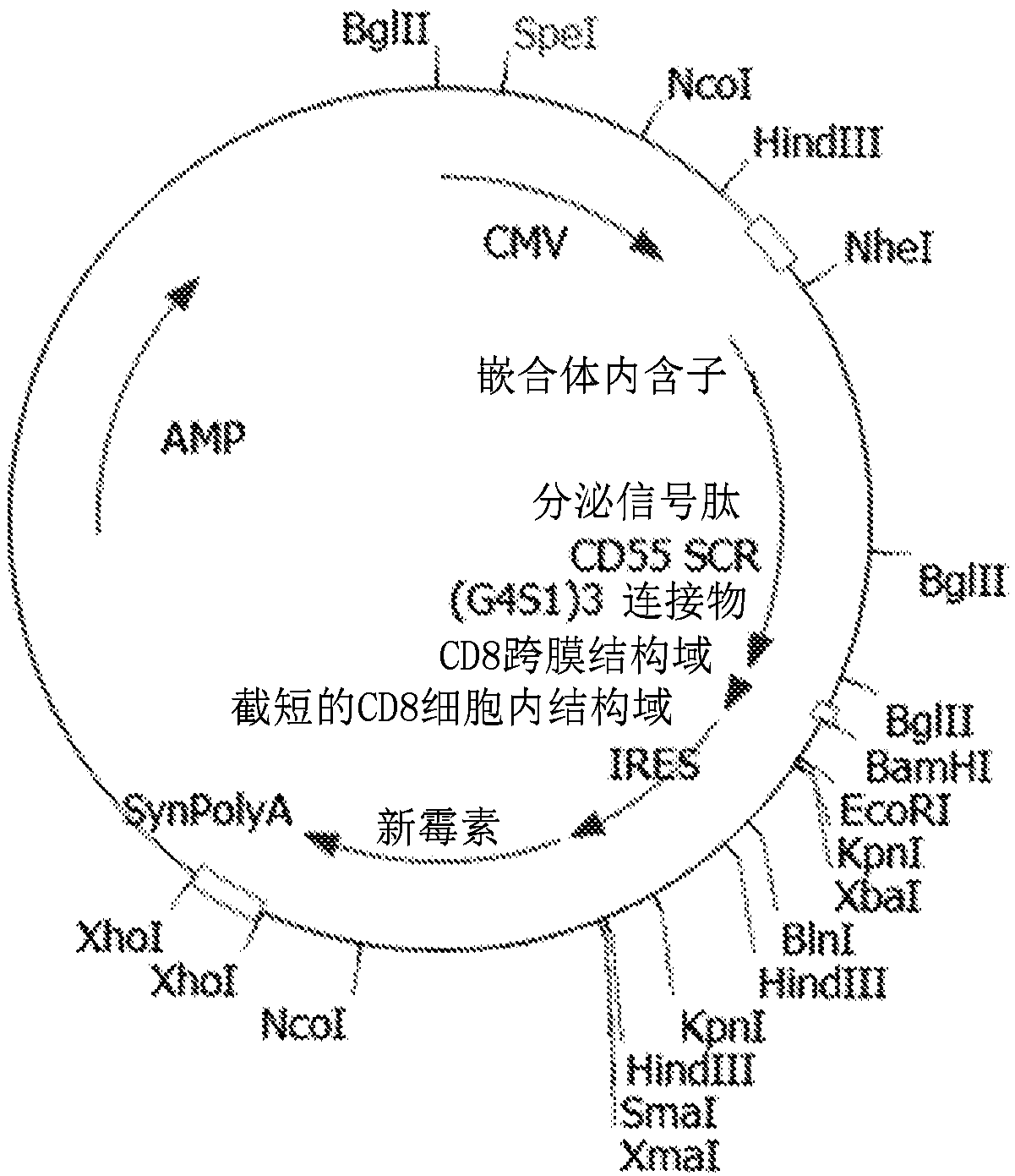
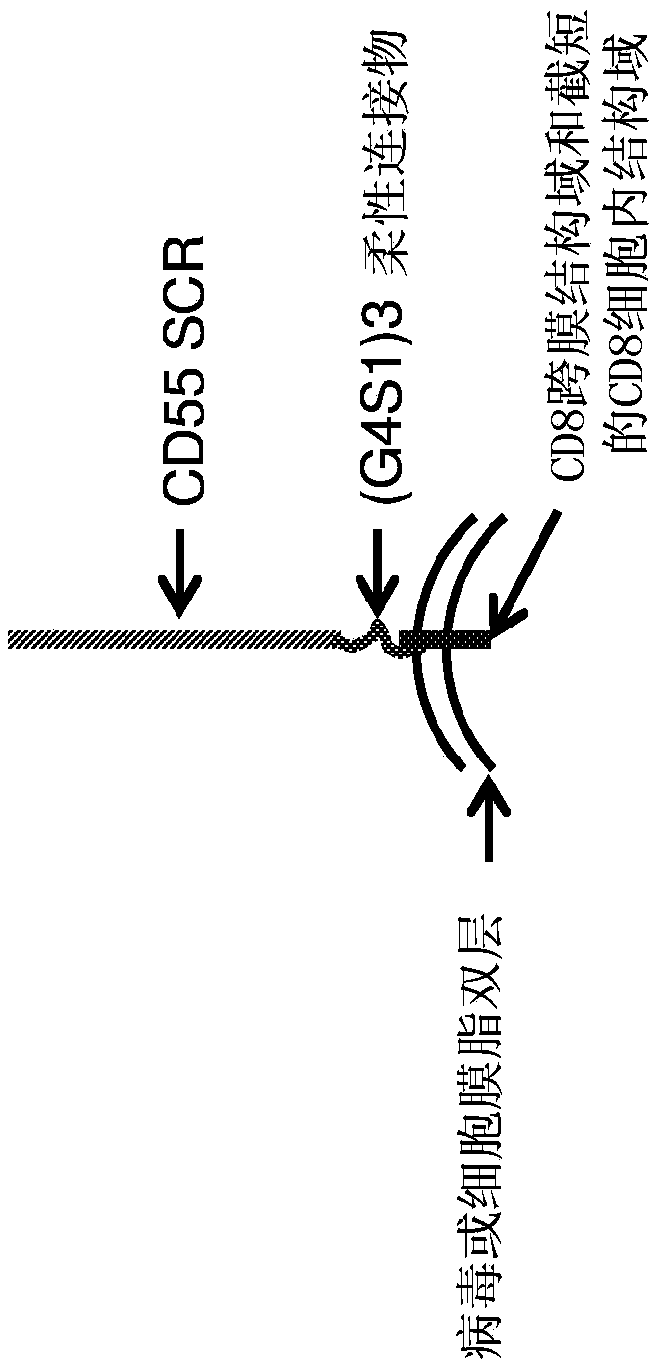
[0035] A modified version of recombinant CD55 was generated with the four short consensus repeats (SCRs) of CD55 downstream of the secretion signal peptide, followed by a flexible linker (3 × G4S1) and the CD8 transmembrane domain and a truncated CD8 intracellular domain ( figure 1 ). The coding sequences were cloned into mammalian expression constructs with CMV promoters (synthetic introns driving expression of recombinant proteins). The expression cassette also contains a drug selectable marker: neomycin phosphotransferase located downstream of the IRES. At the end of the gene expression cassette is a synthetic polyadenylation signal. SEQ ID NO: 1 is the nucleotide sequence of the mammalian cell expression construct. SEQ ID NO: 2 is the amino acid sequence of the expressed protein. When expressed on the cell surface of chicken embryonic fibroblast DF1 or incorporated into the viral membrane, the signal peptide is cleaved, resulting in a mature recombinant fusion protein ...
Embodiment 2
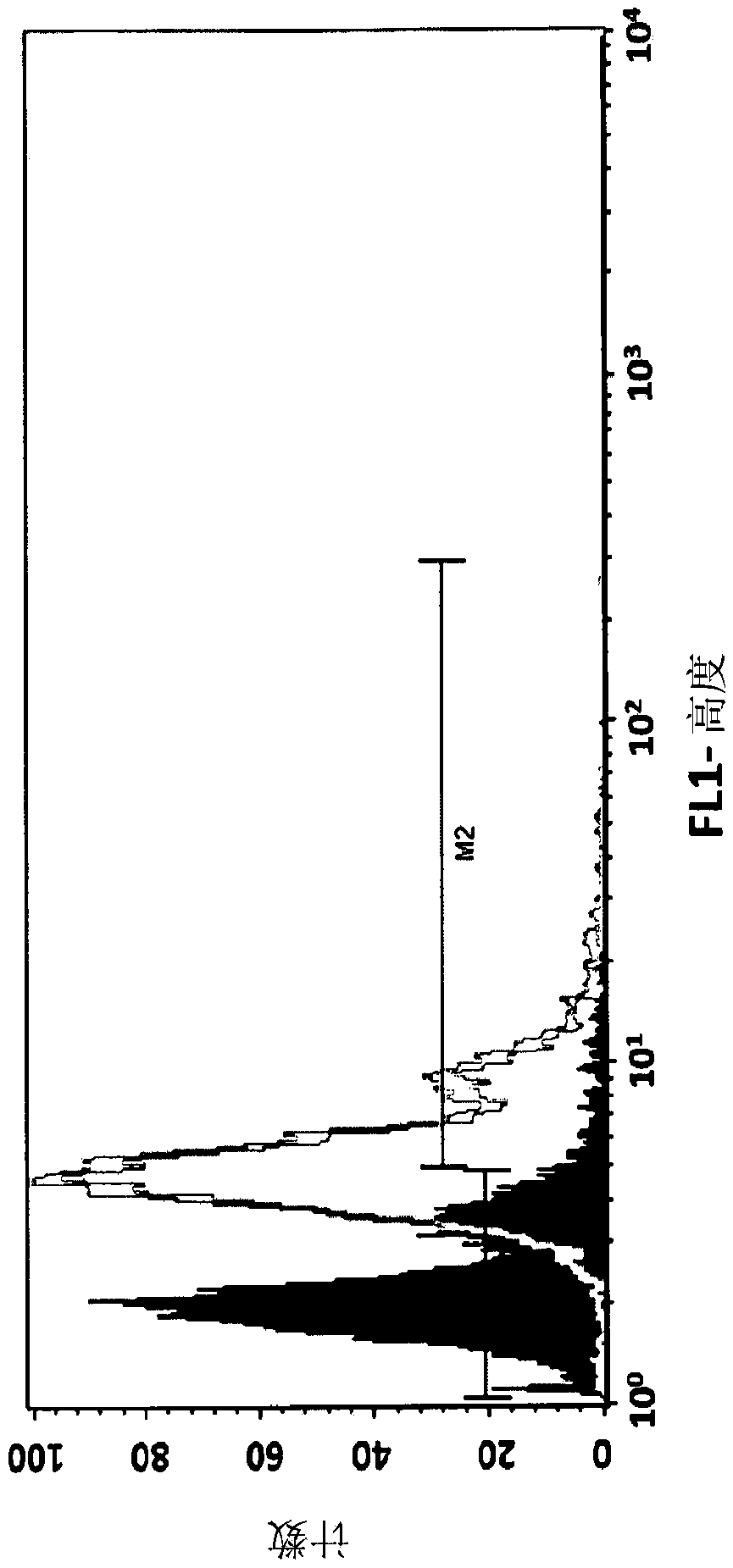
[0037] Mammalian expression constructs were transfected into chicken embryonic fibroblast DF1 cells by PEI 25K (polyethyleneimine, linear 25 kDa, Polysciences, cat# 23966) mediated transfection. 72 hours after transfection, at 300 μg / mLG418 ( aminoglycoside antibiotics) to generate a stable cell line constitutively expressing SEQ ID NO:2. This stable cell line constitutively expressed SEQ ID NO: 3 on its cell surface as detected with a monoclonal antibody specific for mature human CD55 (R&D Systems, catalog # MAB20091). Such as image 3 As shown, recombinant fusion proteins were expressed on DF1 cells stably transfected with constructs encoding recombinant fusion proteins as analyzed by flow cytometry ( image 3 , histogram on the right). Naive DF1 cells served as negative controls ( image 3 , histogram on the left).
Embodiment 3
[0039] A stable cell line expressing SEQ ID NO: 3 on the cell surface was infected with wild-type NDV produced from embryonated chicken eggs. Virus was then titrated on the human tumor cell line HT1080. Equal amounts of virus (measured by PFU) were incubated with 40% normal human serum (NHS) and 40% heat-inactivated normal human serum (iNHS), respectively. Virus surviving incubation with human serum was then scored by plaque assay on HT1080 cells. The ratio of virus recovered after incubation with NHS and with iNHS was calculated. As shown in Table 1, the recovery of virus produced in embryonated eggs was 0.5%, indicating that the vast majority of NDV particles produced by eggs were inactivated, most likely by the human alternative complement pathway. Likewise, the recovery of virus produced by parental chicken embryonic fibroblast DF1 cells was 0.5%. Surprisingly, the recovery rate of virus produced from bulk non-clonal DF1 cells (bulk non-clonal DF1 cells) stably expressi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com