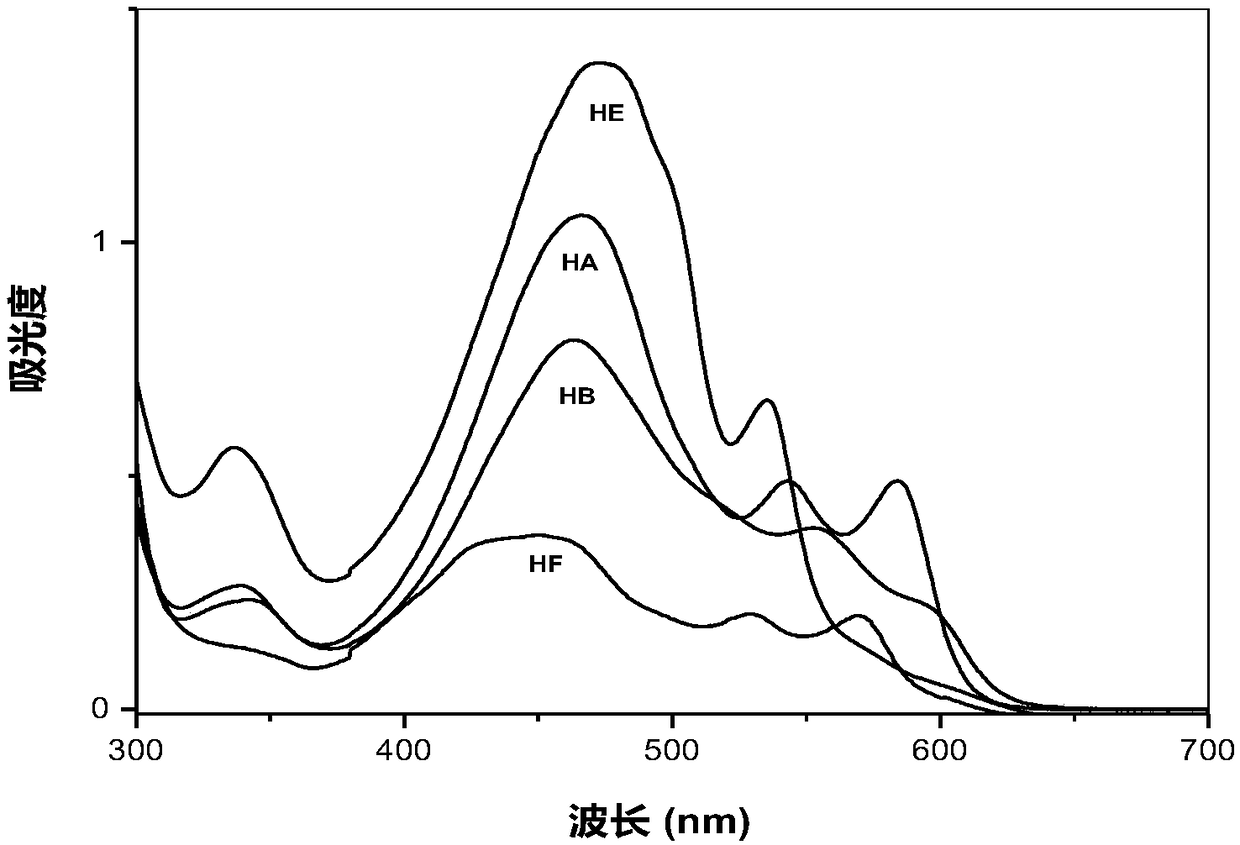
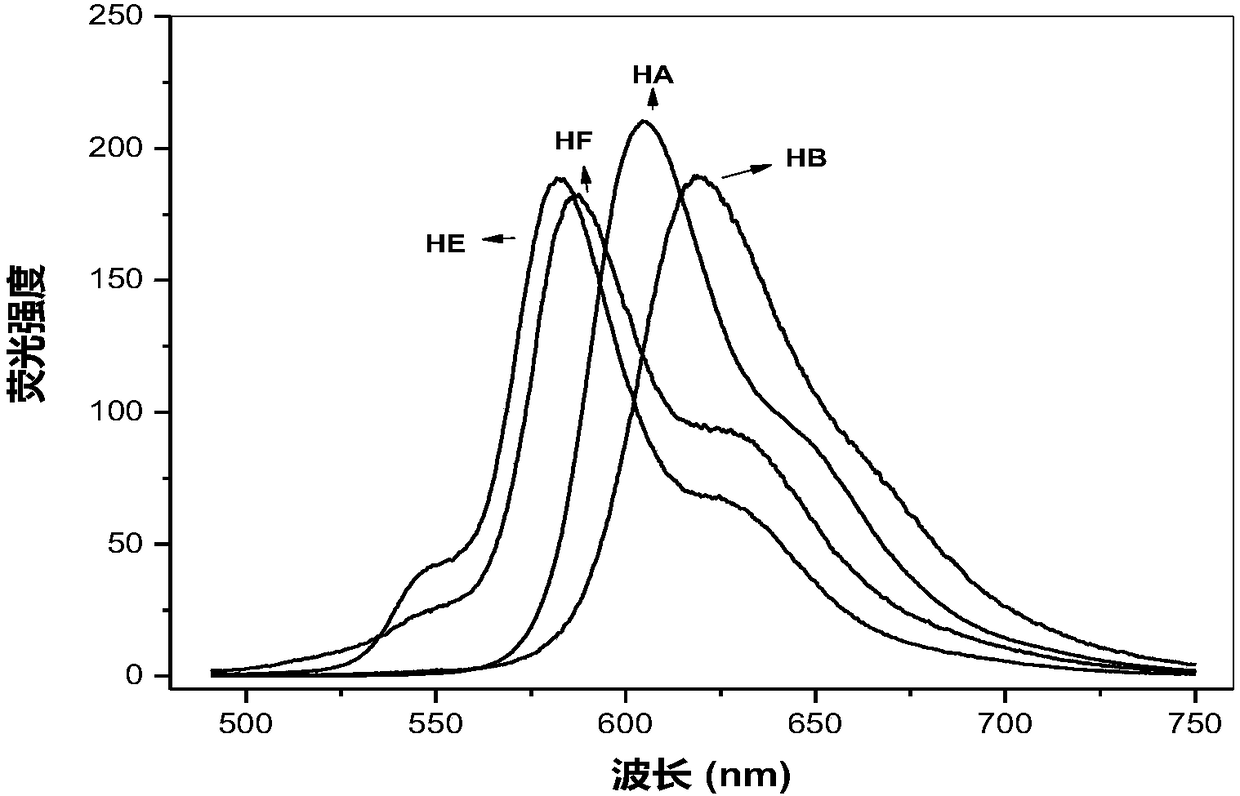
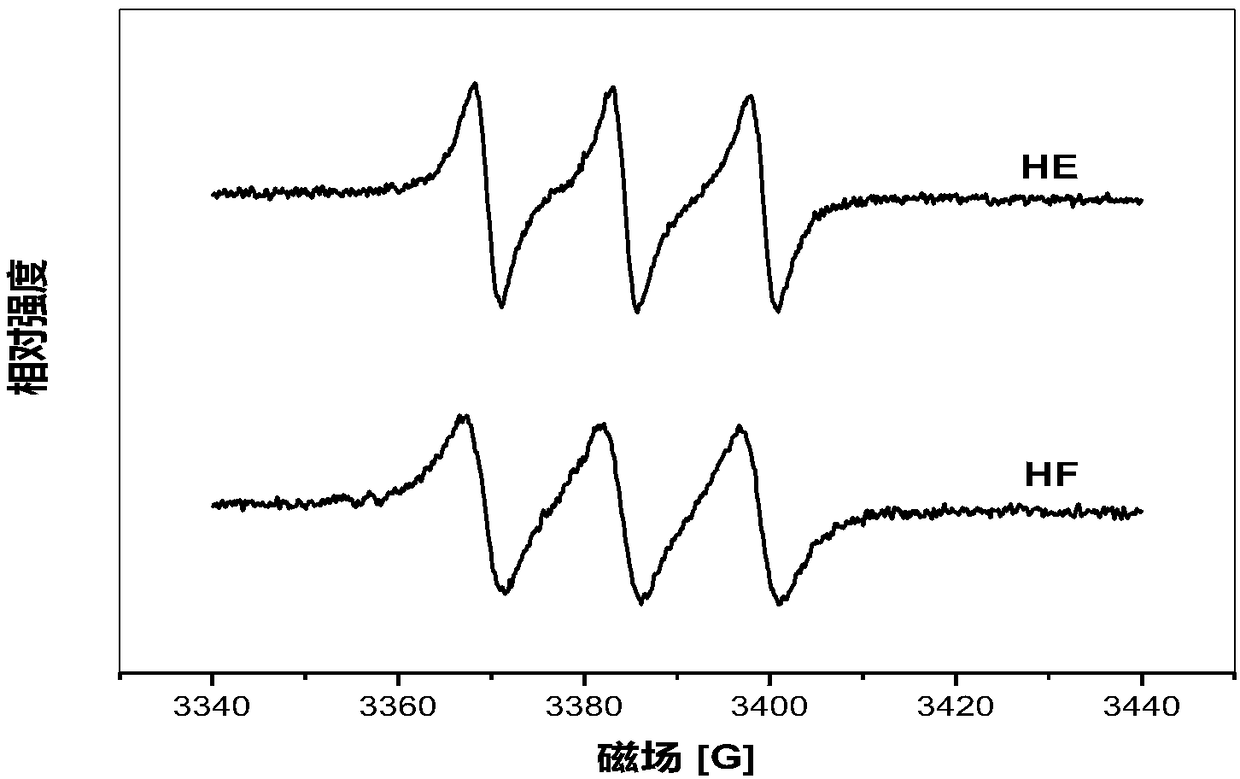
Hypocrellin derivatives, and preparation method and application thereof
A technology of oleocanthal and derivatives, applied in the field of oleocanthin derivatives and preparation thereof, can solve the problems of reduced quantum yield, increased drug cost, low yield of synthetic derivatives and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, the preparation of hypocrellin
[0066] Hypocretin A (HA) 55mg (1.0×10 -4 mol), solid potassium carbonate 120mg (8.7×10 -4 mol) into 15ml of dimethylformamide, mixed thoroughly, heated to 125°C under the protection of nitrogen, reacted with electromagnetic stirring for 4 hours, dried the solvent under reduced pressure, dissolved the residue with an appropriate amount of dilute hydrochloric acid aqueous solution, washed with water until neutral , extracted 3 times with dichloromethane and combined, drained to obtain a black powder, the reaction product was separated and purified on a silica gel thin-layer chromatography plate with 1% citric acid aqueous solution, and the developing solvent was chloroform: ethyl acetate: methanol (V: V: V) = 2:1:1, collect R f =0.84 product, black powder was obtained, which was hypocretin 32 mg, and the yield was 62%.
[0067] The structure detection data of this product are as follows:
[0068] UV spectrum λ max (CHCl ...
Embodiment 2
[0076] Embodiment 2, the preparation of hypocrellin
[0077] Hypocretin A (HA) 50mg (9.2×10 -5 mol), solid sodium carbonate 300mg (2.8×10 -3 mol) into 10ml of dimethylformamide, mixed thoroughly, heated to 130°C under the protection of argon, and reacted with electromagnetic stirring for 4.5 hours. The solvent was dried under reduced pressure, and the residue was dissolved with an appropriate amount of dilute hydrochloric acid aqueous solution, washed with water until medium property, chloroform extraction was combined for 3 times, drained to obtain a black powder, and the reaction product was separated and purified on a silica gel thin-layer chromatography plate with 1% citric acid aqueous solution, and the developing solvent was chloroform: ethyl acetate: methanol (V: V: V) = 2:1:1, collect R f =0.84, the black powder was finally obtained, which was 24 mg of hypocrellin, and the yield was 51%.
[0078] The structure detection data of this product are as follows:
[0079]...
Embodiment 3
[0087] Embodiment 3, the preparation of hypocrellin
[0088] Hypocretin A (HA) 88mg (1.61×10 -4 mol), with solid sodium hydroxide 300mg (5.35×10 -3 mol) into 15ml of dimethylformamide, mixed thoroughly, heated to 130°C under the protection of nitrogen, and reacted with electromagnetic stirring for 4 hours, vacuumed the solvent to dry up, dissolved the residue with an appropriate amount of dilute hydrochloric acid aqueous solution, washed with water until neutral , extracted three times with dichloromethane and combined, and dried to obtain a black powder. The reaction product was separated and purified on a silica gel thin-layer chromatography plate with 1% citric acid aqueous solution, and the developing solvent was dichloromethane:methanol (V:V)=5:1, and R f =0.58 component, 44 mg of black powder was obtained, which was hypocrellin, and the yield was 55%.
[0089] The structural detection data of the product are as follows:
[0090] UV spectrum λ max : 367.0nm, 511.5nm,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nuclear magnetic resonance | aaaaa | aaaaa |
| Nuclear magnetic resonance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com