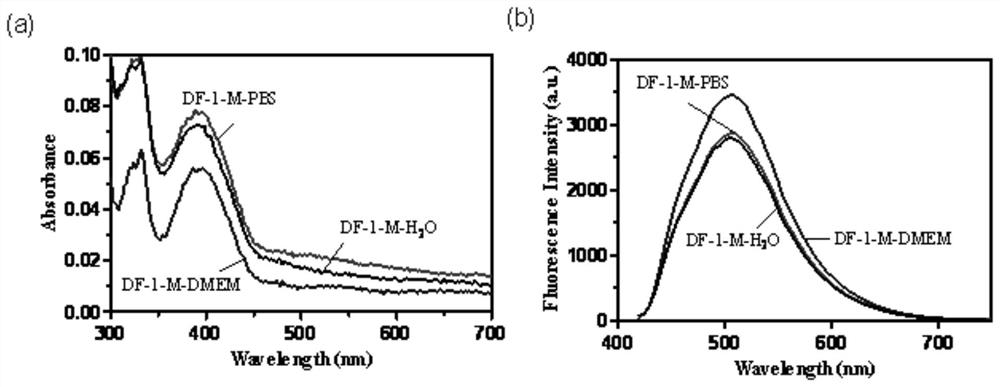
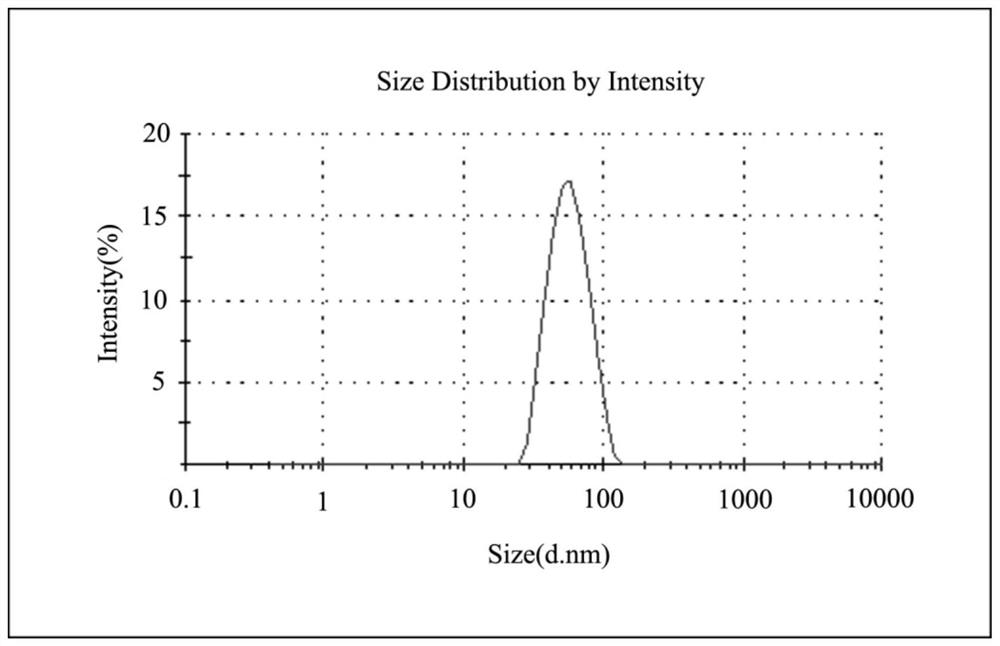
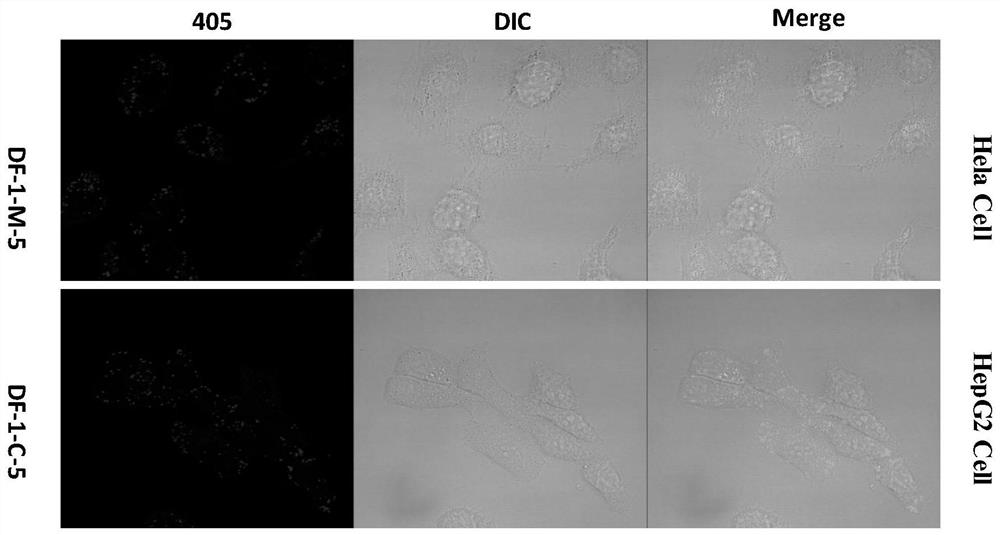
Thermally activated delayed fluorescence (tadf) nanoprobes and their preparation methods and applications in biological imaging
A fluorescent nano-probe, thermal activation delay technology, applied in the field of medicine, can solve the problems of surrounding cell tissue damage, high instrument precision, weak phosphorescence intensity, etc., to reduce self-absorption effect and inner filter effect, reduce background noise, The effect of reducing detection errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Synthesis of Example 1 Compound b
[0060]
[0061] Operation process:
[0062] Compound a (4,5-difluorophthalic anhydride) (300mg) and aniline (186mg) were fed in a mass ratio of 1:1.2, dissolved in 5ml of acetic acid, reacted at 120°C for 4h under the protection of argon, and cooled to room temperature , placed in the refrigerator overnight, crystallized and solids were precipitated, and a white solid was obtained by suction filtration, washed with water, and purified by column to obtain a white powder compound b, 204 mg, with a yield of 57.1%. 1H NMR (400MHz, CDCl3) δ7.79–7.75(t,J=8.0Hz,2H,-Ar),7.54–7.50(m,2H,-Ar),7.44–7.40(m,3H,-Ar). 13C NMR (100MHz, CDCl3) δ165.25, 156.06, 155.91, 153.46, 153.31, 131.32, 129.25, 128.60, 128.45, 126.41, 113.74, 113.67, 113.59, 113.52.
Embodiment 2
[0063] Example 2 Synthesis of Compound DF-1
[0064]
[0065] Operation process:
[0066] Carbazole (130 mg) and NaH (34 mg) were dissolved in THF and stirred at room temperature for 30 min, compound b (100 mg) was added, and the reaction was continued to stir for 2 h, purified by column, and evaporated to dryness to obtain yellow powder compound DF-1, 51 mg, yield 16.3%. 1HNMR (400MHz, Methanol-d4) δ8.25 (d, J = 6.4Hz, 2H, -Ar), 8.17–8.15 (m, 4H, -Ar), 7.97 (d, J = 8.0Hz, 2H, -Ar ),7.57–7.53(m,4H,-Ar),7.49–7.48(m,4H,-Ar),7.47–7.43(m,8H,-Ar),7.39–7.35(m,4H,-Ar), 7.28–7.27(m,3H,-Ar).13C NMR(100MHz,Methanol-d4)δ165.91,139.47,138.85,131.57,131.00,129.34,128.50,126.59,125.97,125.89,124.06,1209.023,12
Embodiment 3
[0067] Synthesis of Example 3 Compound c
[0068]
[0069] Operation process:
[0070]Add compound a (4,5-difluorophthalic anhydride) (1g) and p-aminophenylacetic acid (1.12g) into acetic acid (30mL) and mix and stir, and reflux for 4h. Cool the solution to 4°C overnight, extract and filter 1.32 g of solid compound c was obtained, and the yield was 80%. 1H NMR (400MHz, (CD3)2SO) δ13.14 (s, 1H, -COOH), 8.22 (t, J = 7.6Hz, 2H, -Ar), 8.11–8.08 (m, 2H, -Ar), 7.61 –7.59(m,2H,-Ar).13C NMR(100MHz,(CD3)2SO)δ167.11,165.33,155.04,154.94,153.33,153.23,135.98,130.33,129.40,127.35,114.34,114.30,114.20.1
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com