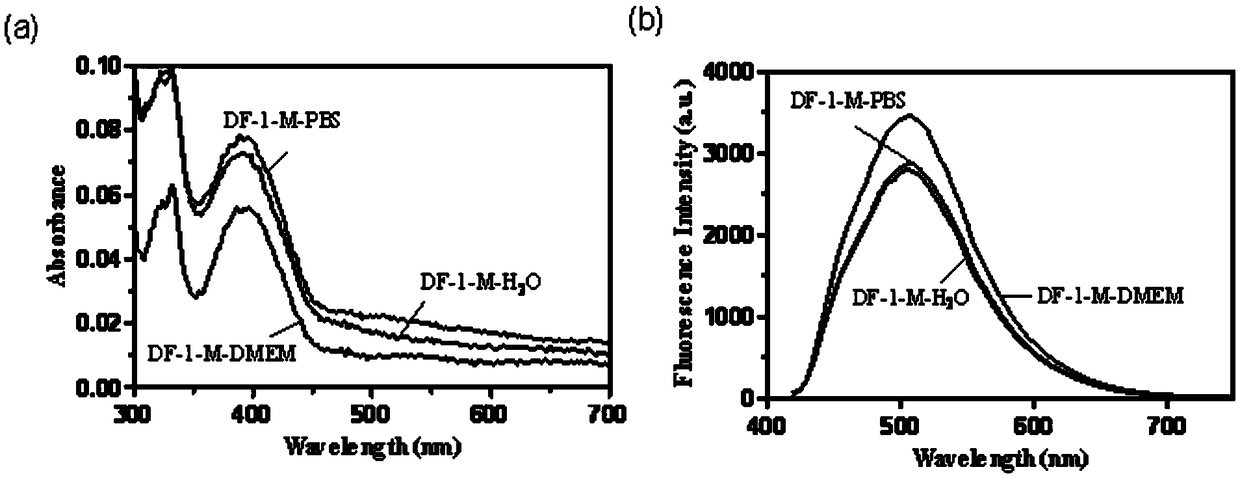
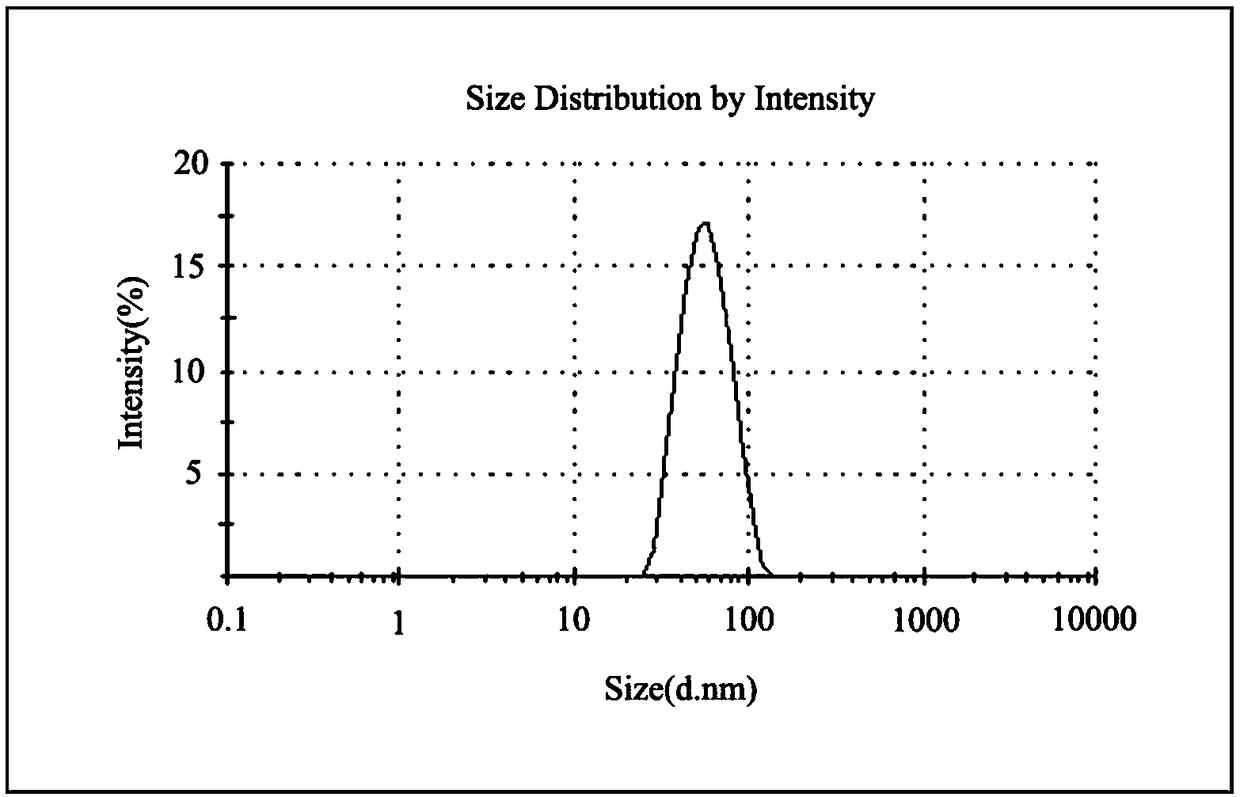
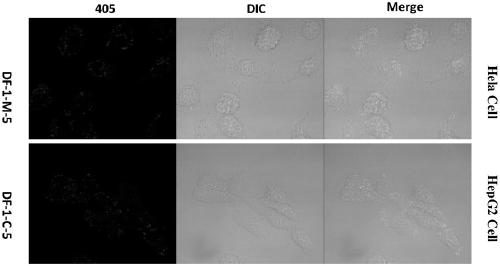
Thermally activated delayed fluorescence (TADF) nanoprobe and preparation method and application thereof in bioimaging
A fluorescent nano-probe and thermal activation delay technology, applied in the field of medicine, can solve the problems of surrounding cell tissue damage, high instrument precision, weak phosphorescence intensity, etc., to reduce self-absorption effect and inner filter effect, reduce background noise, The effect of reducing detection errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The synthesis of embodiment 1 compound b
[0060]
[0061] Operation process:
[0062] Compound a (4,5-difluorophthalic anhydride) (300mg) and aniline (186mg) were fed in a mass ratio of 1:1.2, dissolved in 5ml of acetic acid, reacted at 120°C for 4h under the protection of argon, and cooled to room temperature , placed in the refrigerator overnight, crystallized and solids were precipitated, and a white solid was obtained by suction filtration, washed with water, and purified by column to obtain a white powder compound b, 204 mg, with a yield of 57.1%. 1H NMR (400MHz, CDCl3) δ7.79–7.75(t,J=8.0Hz,2H,-Ar),7.54–7.50(m,2H,-Ar),7.44–7.40(m,3H,-Ar). 13C NMR (100MHz, CDCl3) δ165.25, 156.06, 155.91, 153.46, 153.31, 131.32, 129.25, 128.60, 128.45, 126.41, 113.74, 113.67, 113.59, 113.52.
Embodiment 2
[0063] The synthesis of embodiment 2 compound DF-1
[0064]
[0065] Operation process:
[0066] Carbazole (130 mg) and NaH (34 mg) were dissolved in THF and stirred at room temperature for 30 min, compound b (100 mg) was added, and the reaction was continued with stirring for 2 h, purified by column, and evaporated to dryness to obtain yellow powder compound DF-1, 51 mg, yield 16.3%. 1HNMR (400MHz, Methanol-d4) δ8.25 (d, J = 6.4Hz, 2H, -Ar), 8.17–8.15 (m, 4H, -Ar), 7.97 (d, J = 8.0Hz, 2H, -Ar ),7.57–7.53(m,4H,-Ar),7.49–7.48(m,4H,-Ar),7.47–7.43(m,8H,-Ar),7.39–7.35(m,4H,-Ar), 7.28–7.27(m,3H,-Ar).13C NMR(100MHz,Methanol-d4)δ165.91,139.47,138.85,131.57,131.00,129.34,128.50,126.59,125.97,125.89,124.06,1209.023,12
Embodiment 3
[0067] The synthesis of embodiment 3 compound c
[0068]
[0069] Operation process:
[0070]Compound a (4,5-difluorophthalic anhydride) (1g) and p-aminophenylacetic acid (1.12g) were added to acetic acid (30mL), mixed and stirred, and refluxed for 4h. The solution was cooled to 4°C overnight, extracted and filtered 1.32 g of solid compound c was obtained, and the yield was 80%. 1H NMR (400MHz, (CD3)2SO) δ13.14 (s, 1H, -COOH), 8.22 (t, J = 7.6Hz, 2H, -Ar), 8.11–8.08 (m, 2H, -Ar), 7.61 –7.59(m,2H,-Ar).13C NMR(100MHz,(CD3)2SO)δ167.11,165.33,155.04,154.94,153.33,153.23,135.98,130.33,129.40,127.35,114.34,114.30,114.20.1
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com