Chitin enzyme mutant capable of improving enzyme activity
A technology of chitinase and mutant, applied in the field of enzyme engineering and genetic engineering, can solve the problems of difficult screening, long culture period, loss of enzyme activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 produces chitinase bacterial strain construction
[0030] The chitinase gene fragment chisb (nucleotide sequence shown in SEQ ID NO.9) was chemically synthesized. The PCR conditions were: 98° C. for 3 min, 30 cycles (98° C. for 10 s, 55° C. for 15 s, 72° C. for 1 min), and 72° C. for 5 min. Using pP43NMK as a template and p43-F and p43-R as primers (see Table 1) for PCR amplification of the whole plasmid, a linearized pP43NMK vector was obtained. The PCR conditions were: 98°C for 5min, 25 cycles (98°C for 10s, 55°C for 15s, 72°C for 4min and 30s), 72°C for 5min. After the above amplified products were checked by electrophoresis, the PCR products were purified and recovered using a gel recovery kit. One-step cloning kit clonExpress TM One Step Cloning Kit (Vazyme Biotech Co., Ltd. Nanjing, China), fused the chitinase gene fragment with the linearized vector pP43NMK to obtain the recombinant plasmid pP43NMK-chisb.
[0031] Transform the fused recombinant...
Embodiment 2
[0035] The verification of embodiment 2 chitinase production strains
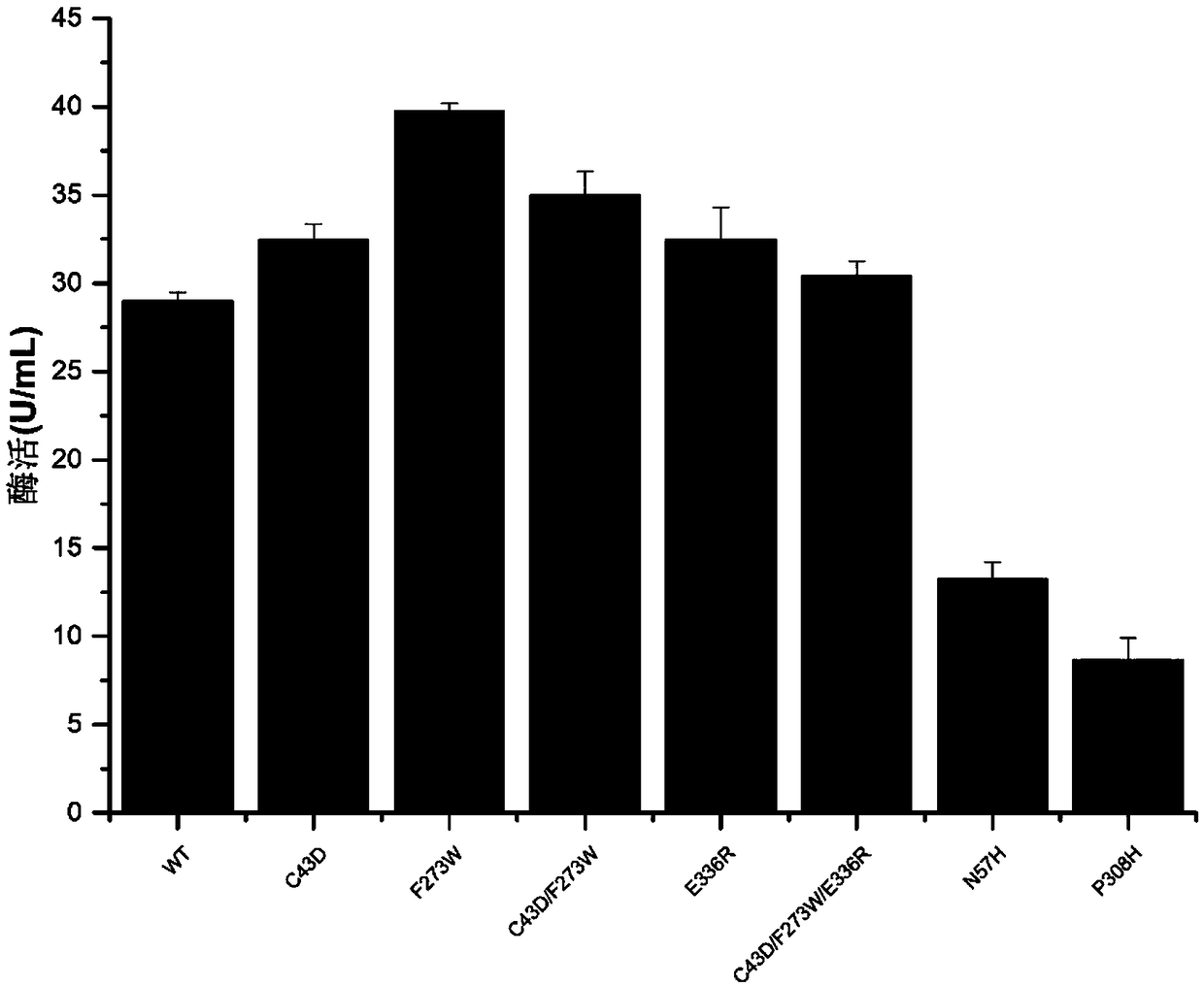
[0036] The plasmid pP43NMK-chisb sequenced correctly in Example 1 was transformed into Bacillus.subtilis WB600. Selected transformants were inoculated into LB medium, cultured at 37°C for 8 hours; transferred to TB medium with an inoculum size of 2%, cultured at 37°C for 12 hours, collected the fermentation supernatant, and detected the enzyme activity of the fermentation supernatant, the results showed , chitinase was secreted to the outside of the cell, and the enzyme activity was 28.98U / mL (results such as figure 1 shown).
Embodiment 3
[0037] The acquisition of embodiment 3 mutant strains
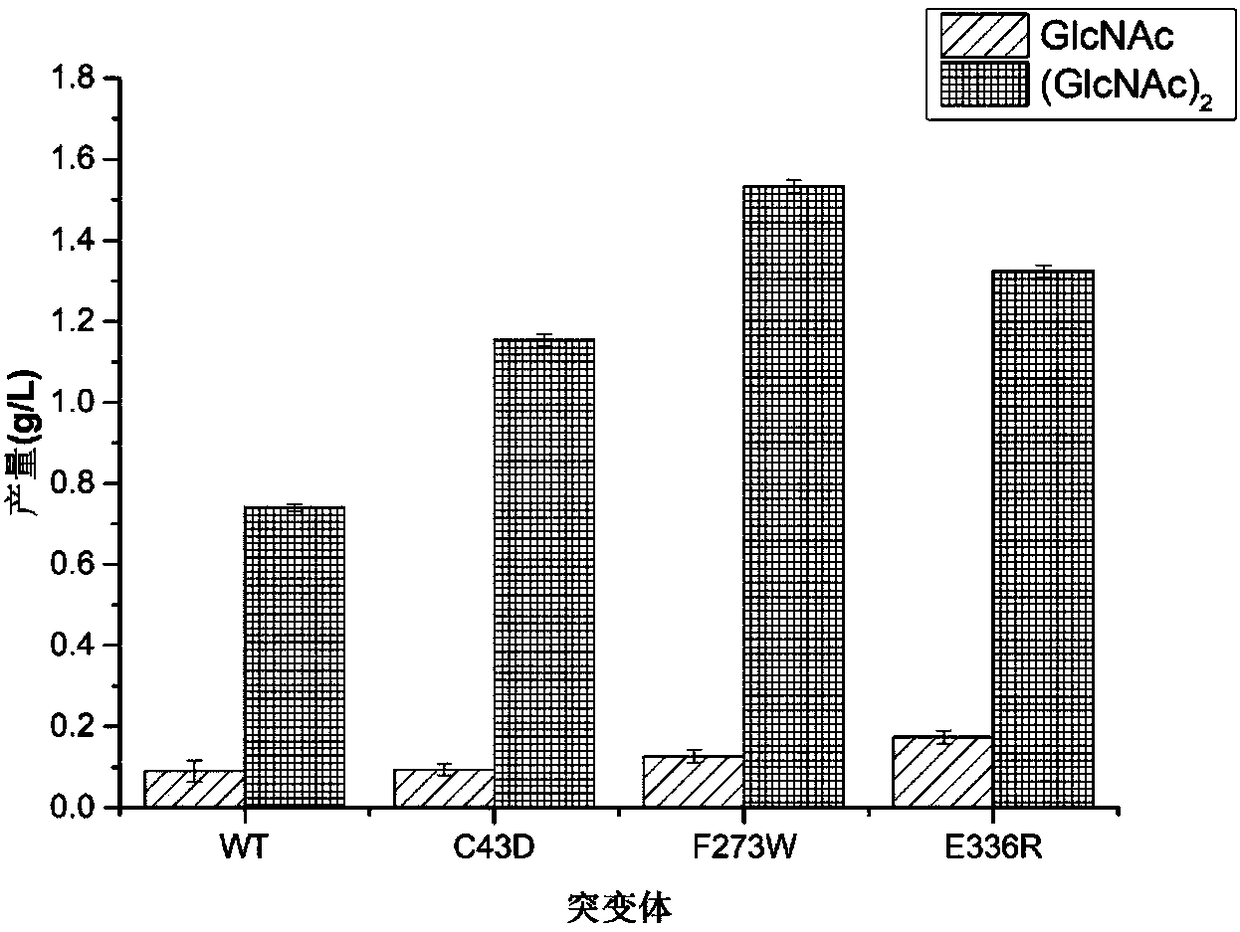
[0038]Use the site-directed mutagenesis kit (purchased from TaKaRa, product number: KM101), design primers (see Table 2), and use the constructed pP43NMK-chisb as a template to carry out PCR, and the 43rd position near the catalytic domain of the chitinase molecule Cysteine is mutated to aspartic acid; 273rd phenylalanine is mutated to tryptophan; or 336th glutamic acid is mutated to arginine, respectively named C43D, F273W, E336R. The PCR reaction conditions were 98°C for 5min, 30 cycles (98°C for 10s, 55°C for 15s, 72°C for 4min30s), and 72°C for 5min. The gel recovery kit was used to purify and recover the PCR products, and the recovered products were checked by electrophoresis. The product was transformed into E.coli JM109 and sequenced by Tianlin (Wuxi) Co., Ltd., and the transformants were named pP43NMK-chisb-C43D, pP43NMK-chisb-F273W, and pP43NMK-chisb-E336R.
[0039] Using the plasmid of the correctly sequence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com