Intracellular micro-RNA non-enzymatic amplification detection method based on electrostatic affinity nano-transporter and cell imaging
A non-enzymatic amplification and cell imaging technology, applied in biochemical equipment and methods, microbial measurement/testing, etc., can solve problems such as sensitivity limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Synthesis of Embodiment 1 Nano Gold Particles (AuNPs)
[0095] When synthesizing AuNPs, positively charged poly-L-lysine (PLL) was used as a capping agent and a reducing agent, and PLL reduced gold salt ions (AuCl 4 -1 ), the metal ions induce the oxidation of the amine to nitrite, a direct redox reaction occurs, and AuNPs are spontaneously formed, and the PLL is encapsulated on the surface of the AuNPs to stabilize the formation of nanoparticles. The stabilization of the resulting AuNPs is ensured by a combination of steric and electrostatic interactions of charged peptides. First, dissolve 30mg of PLL in 3mL of water, shake and mix for 5 minutes to dissolve it completely, and prepare a PLL solution with a concentration of 10mg / mL; take 10uL of chloroauric acid solution with an original concentration of 1.52mol / L, add it to 142uL of water, and mix well. Mix well, avoid light, and prepare a chloroauric acid solution with a concentration of 0.1mol / L; finally add nuclea...
Embodiment 2
[0096] Example 2 Establishment of microRNA non-enzymatic amplification platform based on electrostatic affinity nanotransporter and its sensitivity experiment
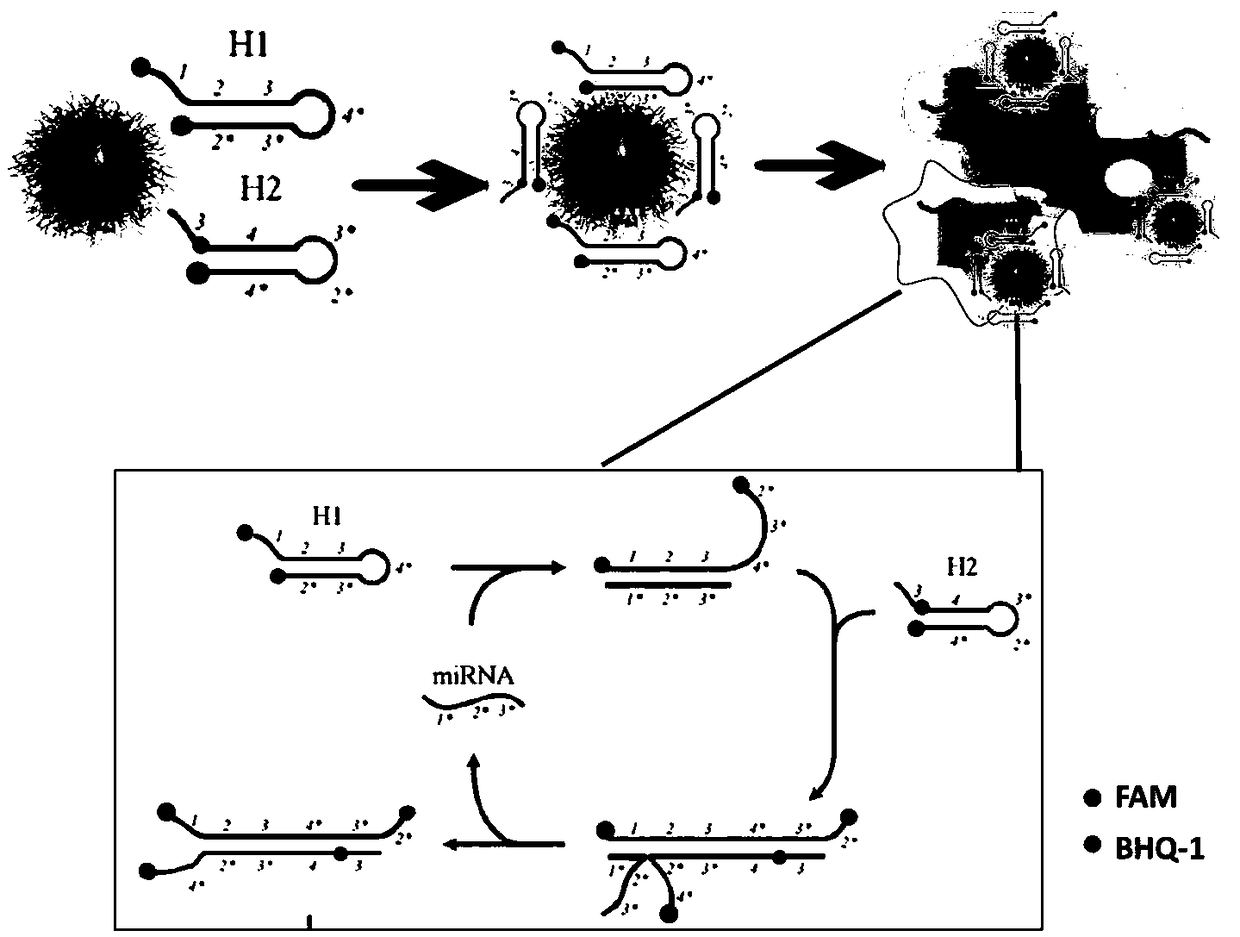
[0097]According to the principle of the microRNA non-enzymatic amplification platform based on the electrostatic affinity nanotransporter, the hairpin probes H1 and H2 required for the non-enzymatic amplification system were designed for the microRNA21 sequence, and their sequences are shown in Table 1. The hairpin probe H1 adopts a double-headed labeling strategy, and its 5' end and 3' end are respectively labeled with a fluorescent group FAM and a quencher group BHQ-1; the 3' end of H2 is labeled with a fluorescent group FAM, and the 5' end The 8 base T marks BHQ-1. The hairpin probes were all synthesized by Huada Gene Technology Co., Ltd., and H1 and H2 were dissolved in TE buffer (final concentration 100 μM, stored in -20 refrigerator).
[0098] Used sequence in the embodiment of table 1
[0099]
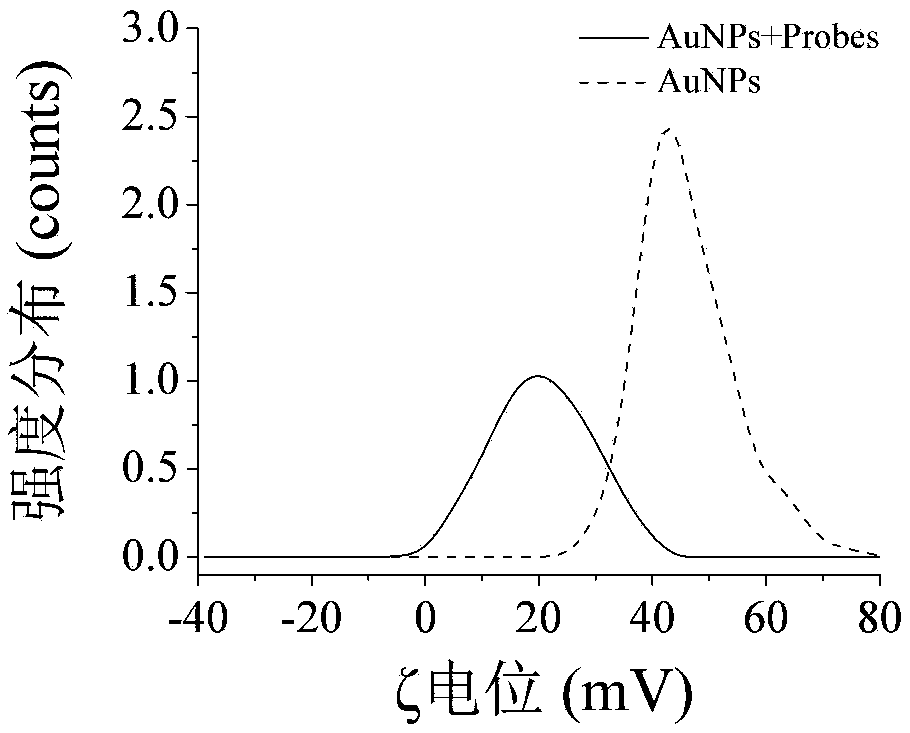
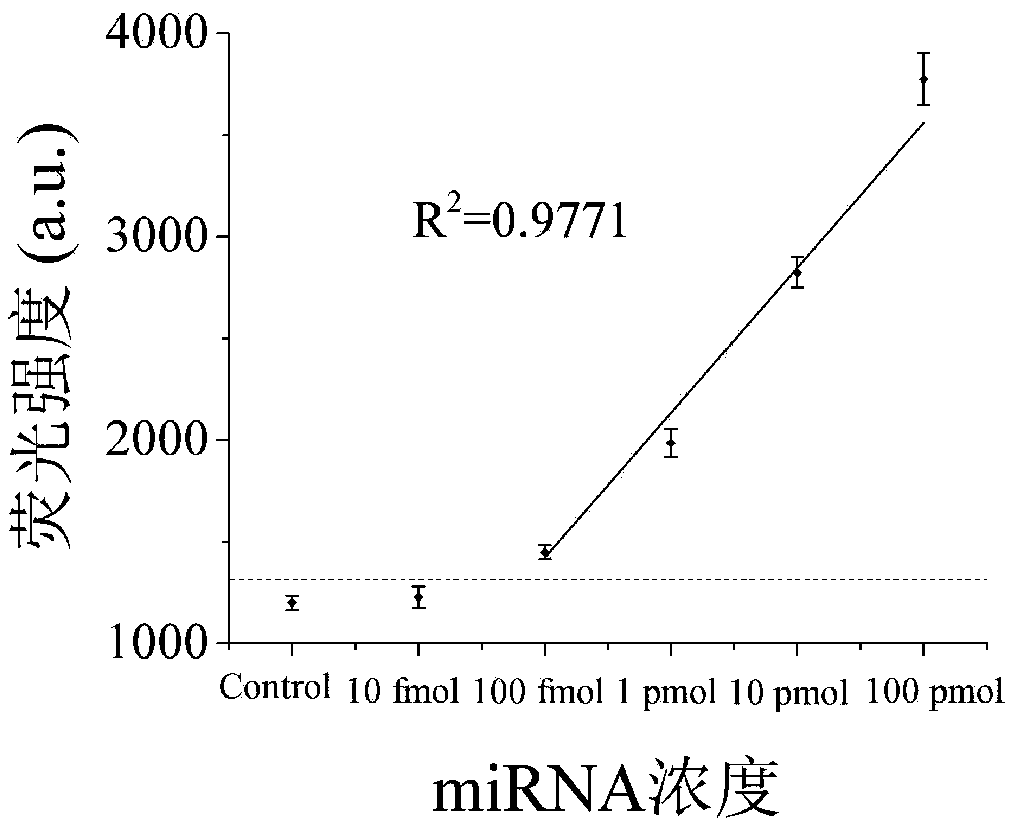
[0100] In o...
Embodiment 3
[0103] Example 3 Specific verification of the intracellular microRNA non-enzymatic amplification platform based on electrostatic affinity nanotransporter
[0104] In order to verify the specificity of the intracellular microRNA non-enzymatic amplification platform based on the electrostatic affinity nanotransporter, microRNA210 and microRNA214, which have a base sequence similar to microRNA21, were added to the platform respectively, and the amount added was 10 pmol respectively, and the fluorescent signal was detected. Experimental results such as Figure 4 As shown, the Control group is a blank control. The fluorescence intensity values of the microRNA210, microRNA214 and Control control groups at the emission wavelength of 518nm are 801.775a.u., 788.7a.u., and 739.5a.u., respectively, and the fluorescence intensities of these three groups are basically the same; The fluorescence intensity value at the wavelength of 518nm was 3763a.u., and a strong fluorescence signal was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com