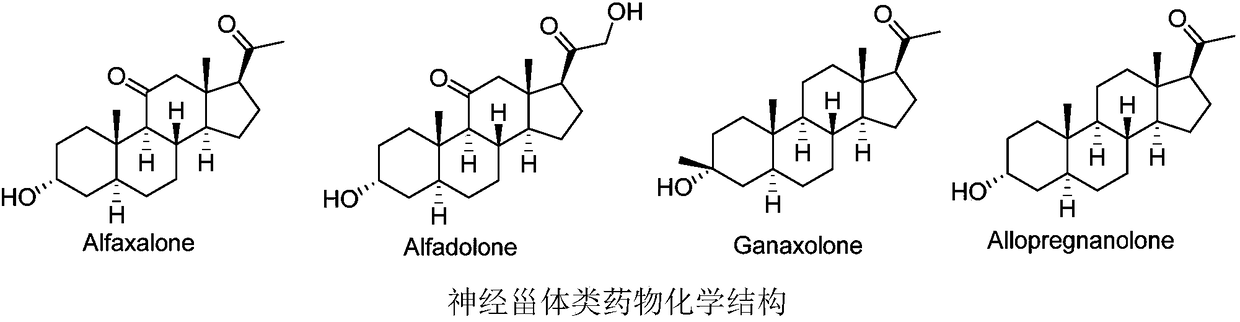
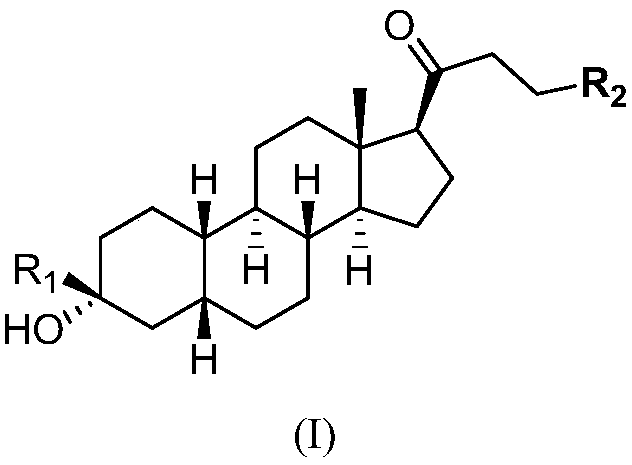
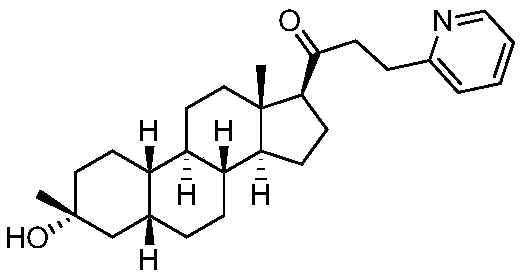
Novel GABA (gamma-aminobutyric acid) receptor modulator and application thereof
A pharmacy and compound technology, applied in the field of new regulators, can solve the problems of strong lipophilicity and low oral bioavailability, and achieve good biological activity and reduce the effect of metabolic rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1. Preparation of 1-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxyl-3,13-dimethylhexadecahydro-1H-cyclopentyl[a]phenanthrene -17-position)-3-(pyridine-2-position) propyl-1-one (1-1)
[0055]
[0056] Compound 1-1 was prepared according to the following process:
[0057]
[0058] Dissolve commercially available compound A (100mg, 0.31mol) in ethanol (5ml) and water (2ml), add pyridine-2-carboxaldehyde (68mg, 0.62mmol) and sodium hydroxide (18mg, 0.46mmol) successively, room temperature Stirred for 15 hours, concentrated and extracted twice with EA (30mlx2), washed once with brine (30ml), dried over anhydrous sodium sulfate, concentrated and silica gel column chromatography to obtain 72mg of white foamy solid B, yield: 57%. 1 H-NMR (400MHz, CDCl 3 )δ: 8.67(d, 1H, J=3.2Hz), 7.77-7.73(m, 1H), 7.54-7.46(m, 2H), 7.33-7.28(m, 2H), 2.92(t, 1H, J= 8.4Hz),2.37-2.29(m,1H),2.06-1.99(m,3H),1.71-1.56(m,14H),1.46-1.33(m,8H),1.25-1.16(m,10H),1.10 -1.00(m,3H),0.64(s,3H); ESI...
Embodiment 2
[0064] Example 2. Preparation of 1-(3-((3R,5R,8R,9R,10S,13S,14S,17S)-3-hydroxyl-3,13-dimethylhexadecahydro-1H-cyclopentyl[ a] phenanthrene-17-position)-3-oxo)-1H-pyrazole-4-carbonitrile (2-1)
[0065]
[0066] Compound 2-1 was prepared according to the following process:
[0067]
[0068] Compound A (50mg, 0.15mmol) was dissolved in DME (20ml), paraformaldehyde (9mg, 0.29mmol) and dimethylamine hydrochloride (21mg, 0.26mmol) were added successively, and the mixture was refluxed for 48 hours. Adjust the pH to 13-14 with 2M NaOH aqueous solution, extract three times with EA (30mlx3), dry over anhydrous sodium sulfate, concentrate and column chromatography to obtain 19 mg of yellow oily liquid C, yield: 32%. ESI-MS m / z 376.2(M+H) + .
[0069] Dissolve compound C (130mg, 0.34mmol) in THF (10ml), cool in an ice bath to 0-5°C, add iodomethane (246mg, 1.73mmol), continue the reaction at 0-5°C for 1 hour, and Stir overnight. Concentrate to dryness to obtain 145 mg of yellow...
Embodiment 3
[0075] Example 3. Activity Test
[0076] Compounds were tested for modulatory activity using an artificial patch clamp method using ex vivo electrophysiology. Using stable expression of human GABA A HEK293T cell line of the receptor (subunit composition: α1β2γ2). Methods as below:
[0077] with GABA A The receptor subunit α1β2γ2 was stably transfected into HEK293T cells, the cells were passaged, and then inoculated into sterile culture dishes. Confluent populations of cells are electrocoupled and cells are cultured at a density capable of recording single cells. Total cell currents were measured using a HEKA EPC-10 amplifier and using PatchMaster software. Cells were voltage clamped at -80 mV clamping voltage. After pre-incubation with increasing concentrations of the test article, GABA receptors were stimulated with 2uM GABA. The pre-incubation time is 30s, the stimulation time is 2s, and the dilution concentration of the test product is 0.01, 0.1, 1, 10uM.
[0078] D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com