A kind of carboxy-containing coumarin derivative and its synthesis method and application
The technology of a coumarin derivative and a synthesis method is applied in the field of coumarin derivatives and achieves the effects of good selectivity, broad application prospects and simple detection means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
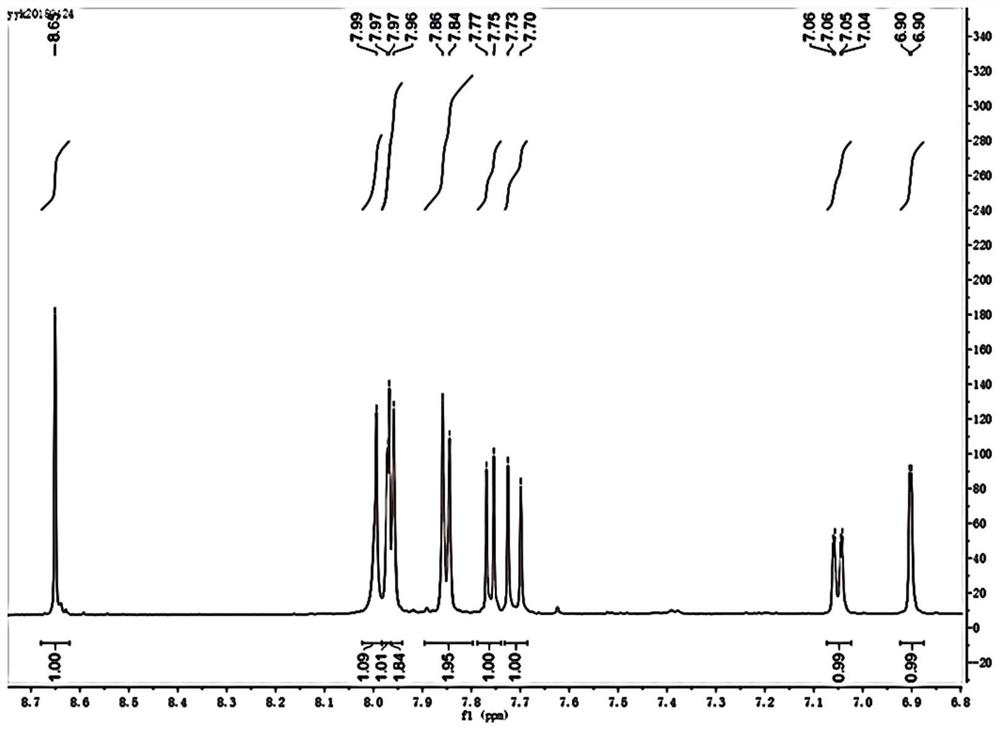
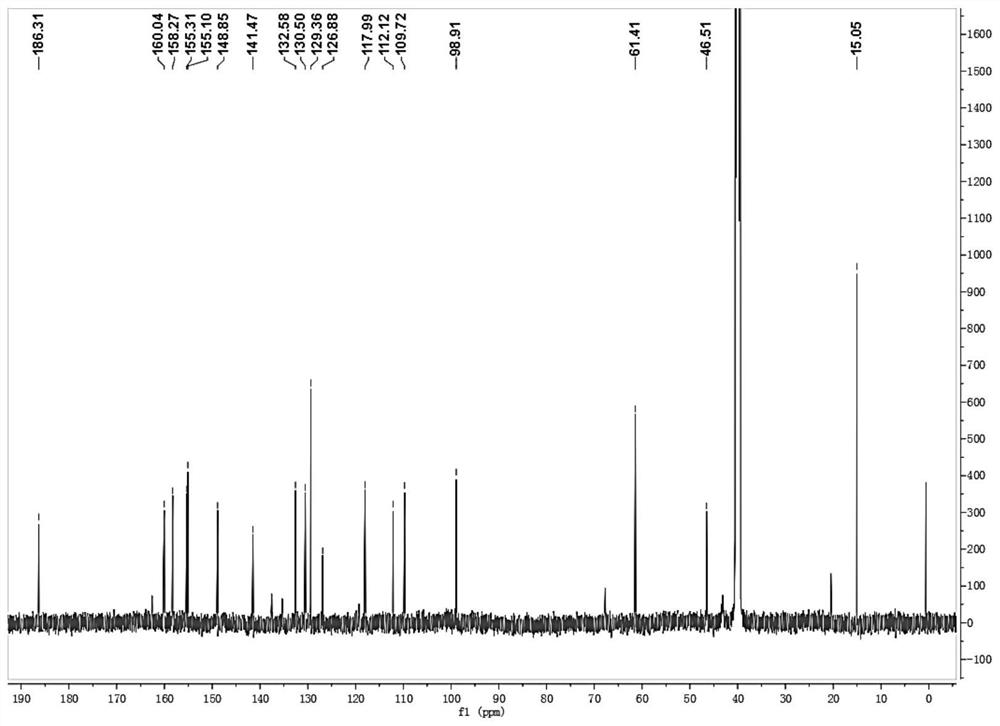
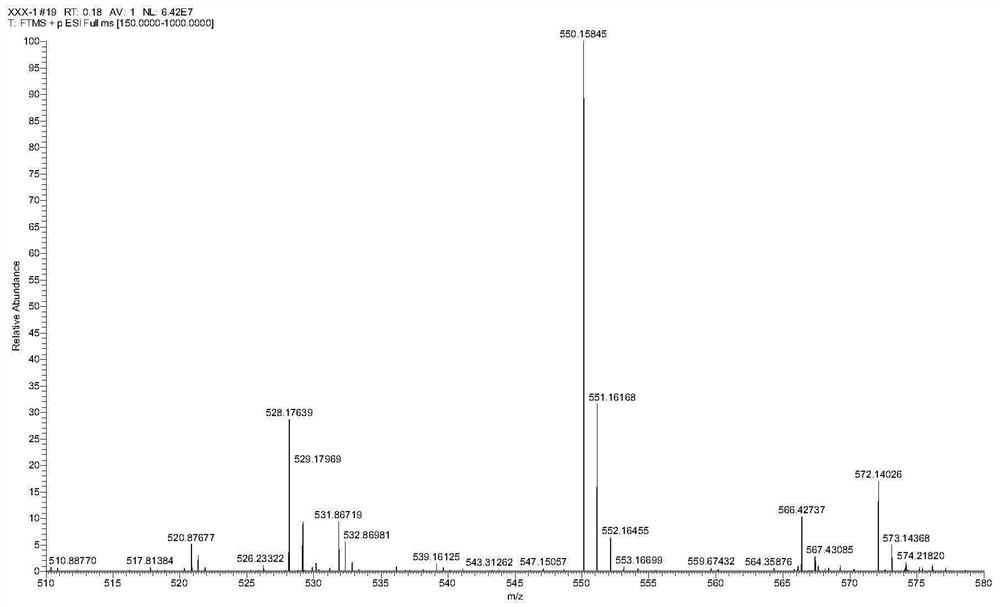
[0048] Preparation and characterization of XI-2
[0049]1) Ethyl chloroformate (0.488g, 4.50mmol) and sodium bicarbonate (0.315g, 3.75mmol) were dissolved in 10mL of tetrahydrofuran, and slowly added dropwise to the mixture containing m-hydroxyphenylpiperazine (0.535 g, 3.00mmol) in a volume ratio of 1:1 tetrahydrofuran and deionized water solution (40mL) in a round bottom flask, stirred overnight at room temperature; after the reaction was completed, extracted with dichloromethane, dried over anhydrous sodium sulfate, and evaporated under reduced pressure White needle crystals A: ethyl 4-(3-hydroxyphenyl)piperazine-1-carboxylate were obtained.
[0050] 2) Slowly add phosphorus oxychloride (5mL) dropwise into 10mL N,N-dimethylformamide in an ice-water bath, and after stirring for 30min, dissolve 0°C in 10mL N,N-dimethylformamide A (0.500g, 2.00mmol) in methyl formamide was slowly added to the above solution, and stirred for 2h after mixing completely; the system was introduce...
Embodiment 2
[0056] Prepare PBS buffer solution with pH=7.4 and concentration of 10mM, prepare 2mM XI-2 DMSO solution, prepare 20mM cysteine aqueous solution; take 2mL of PBS-DMSO (10mM, pH=7.4, 4:1, v / v ) solution and 10 μL XI-2 DMSO solution were added to the fluorescence cuvette, and the volume of cysteine solution was gradually added to 0, 5, 10, 15, 20, 25, 30, 35, 40, 45 μL, and at the same time The fluorescence intensity at 500nm measured by the fluorescence spectrometer was 50.23, 898.8, 1590, 1917, 2360, 2567, 2754, 2952, 2962, 3081, and the fluorescence intensity gradually increased. Fluorescence emission map see Figure 4 .
Embodiment 3
[0058] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 10 mM, prepare a 2 mM XI-2 DMSO solution, prepare a 0.2 M cysteine aqueous solution, and prepare a 0.2 M N-ethylmaleimide (NEM) solution; Add 10μL of XI-2 DMSO solution to 2mL PBS-DMSO (10mM, pH=7.4, 4:1, v / v) fluorescence cuvette, take 10μL of cysteine solution, add to the above buffer solution, When the fluorescence intensity of the system no longer changes, take 0.5 μL NEM solution and add it to the above solution. When the fluorescence intensity of the system no longer changes, continue to add 2 μL NEM solution. When the fluorescence intensity of the system no longer changes, add 2 μL NEM solution was used 4 times until the fluorescence intensity of the system did not change; adding 10 μL of cysteine solution again made the fluorescence of the system increase again. Fluorescence emission map see Figure 5 . Take another fluorescence cuvette, add 10μL XI-2 DMSO solution to 2mL PBS-DMSO (10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com