Application of flavonoid components to prepare pharmaceutical preparation for reducing enterogenous urotoxin or precursor molecules
A technology of pharmaceutical preparations and intestinal source urine, which is applied in the field of medicine, can solve the problems of low dialysis efficiency and filtration efficiency, inability to effectively remove protein-bound uremic toxins, and low clearance rate of protein-bound uremic toxins, and achieve reduced production , Significant kidney damage protection, reduce the effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
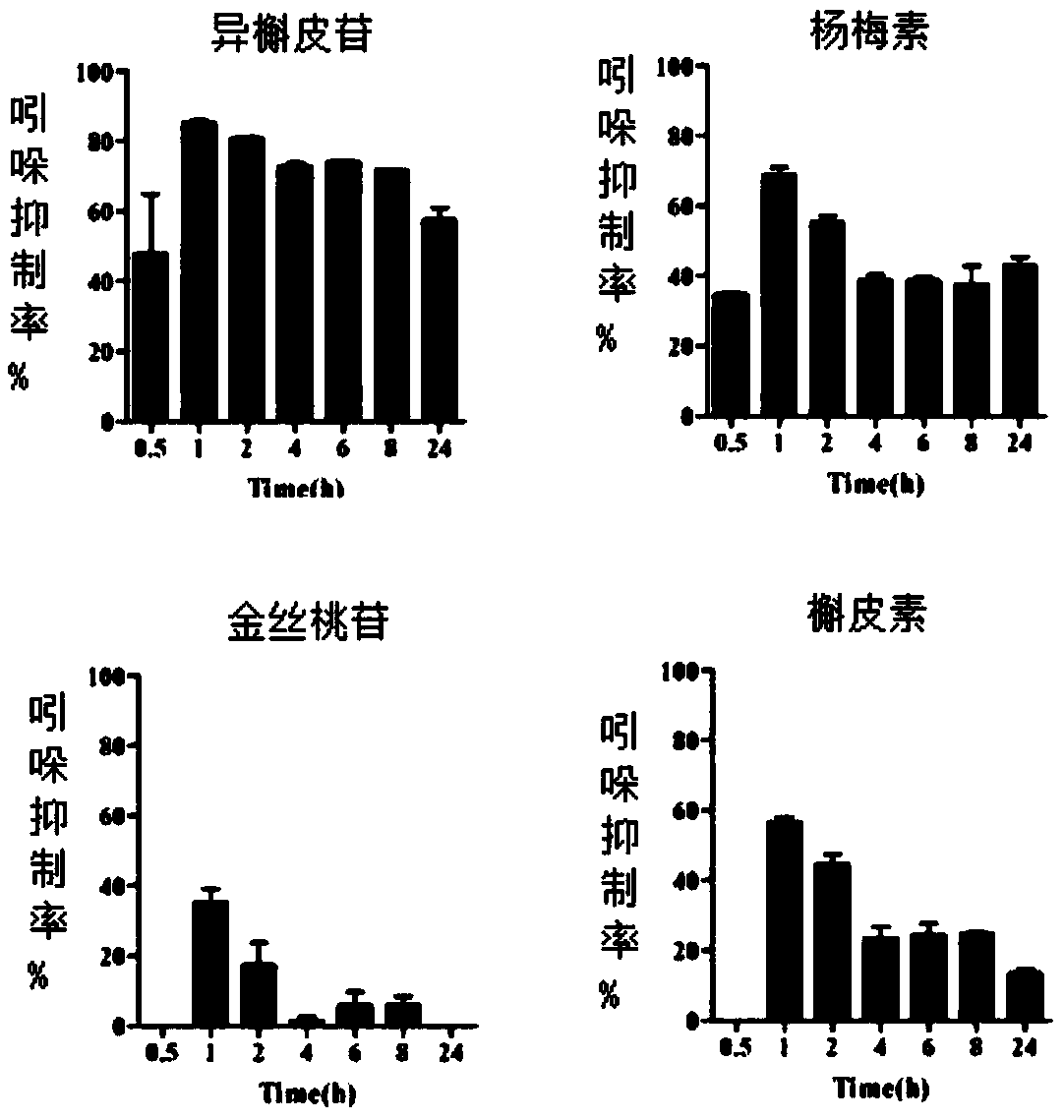
[0019] Example 1 Flavonoids reduce the experiment of model bacteria Escherichia coli to synthesize indole
[0020] 1. Experimental method
[0021] 1.1 Preparation of pharmaceutical compounds
[0022] Accurately weigh a certain amount of isoquercitrin (abbreviated Iso) standard substance, myricetin (abbreviated Myr) standard substance, quercetin (Hyp) standard substance, hyperoside (Que) standard substance, and add appropriate amount of DMSO to prepare Prepare the stock solution at the concentration required for the experiment and store it in a -20°C refrigerator for later use.
[0023] 1. Preparation of 2L-B medium
[0024] Accurately weigh 1 g of tryptone, 1 g of sodium chloride, and 0.5 g of yeast extract powder. Add 100ml of ultrapure water, stir to dissolve until clear and transparent without precipitation, sterilize it in a high-pressure steam sterilizer for 2 hours, and take it out of the refrigerator stored at 4°C for later use.
[0025] 1.3 Activation, dilution and...
Embodiment 2
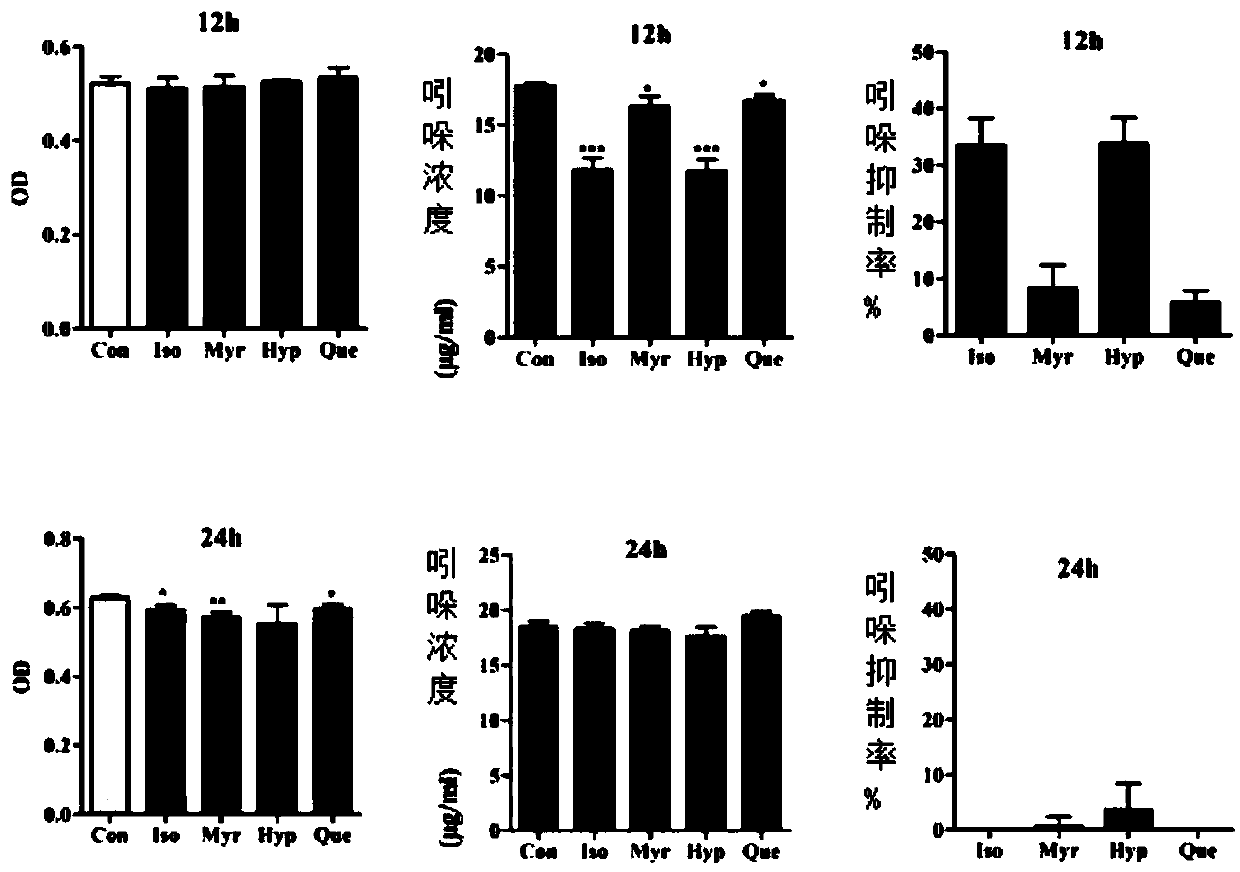
[0037] Example 2 The experiment of flavonoids reducing the synthesis of indole by mixed bacteria in the cecum of rats
[0038] 1. Experimental method
[0039] With embodiment 1.
[0040] 2. Experimental results
[0041]Select four compounds, isoquercitrin, myricetin, hyperin, and quercetin, and give the compounds with a final concentration of 200 μM. After anaerobic culture with rat cecal bacteria for 12 and 24 hours, HPLC detects indole in the bacterial supernatant. content, and compared the inhibitory effects of the four compounds at a concentration of 200 μM on indole secretion.
[0042] The results showed that the four compounds of isoquercitrin, myricetin, hyperoside and quercetin could inhibit bacterial secretion of indole to varying degrees. Among the four compounds, isoquercitrin has the strongest inhibitory effect on indole secretion (eg figure 2 ). The four compounds co-cultured with the bacteria for 12 hours had no significant effect on the growth of the bacte...
Embodiment 3
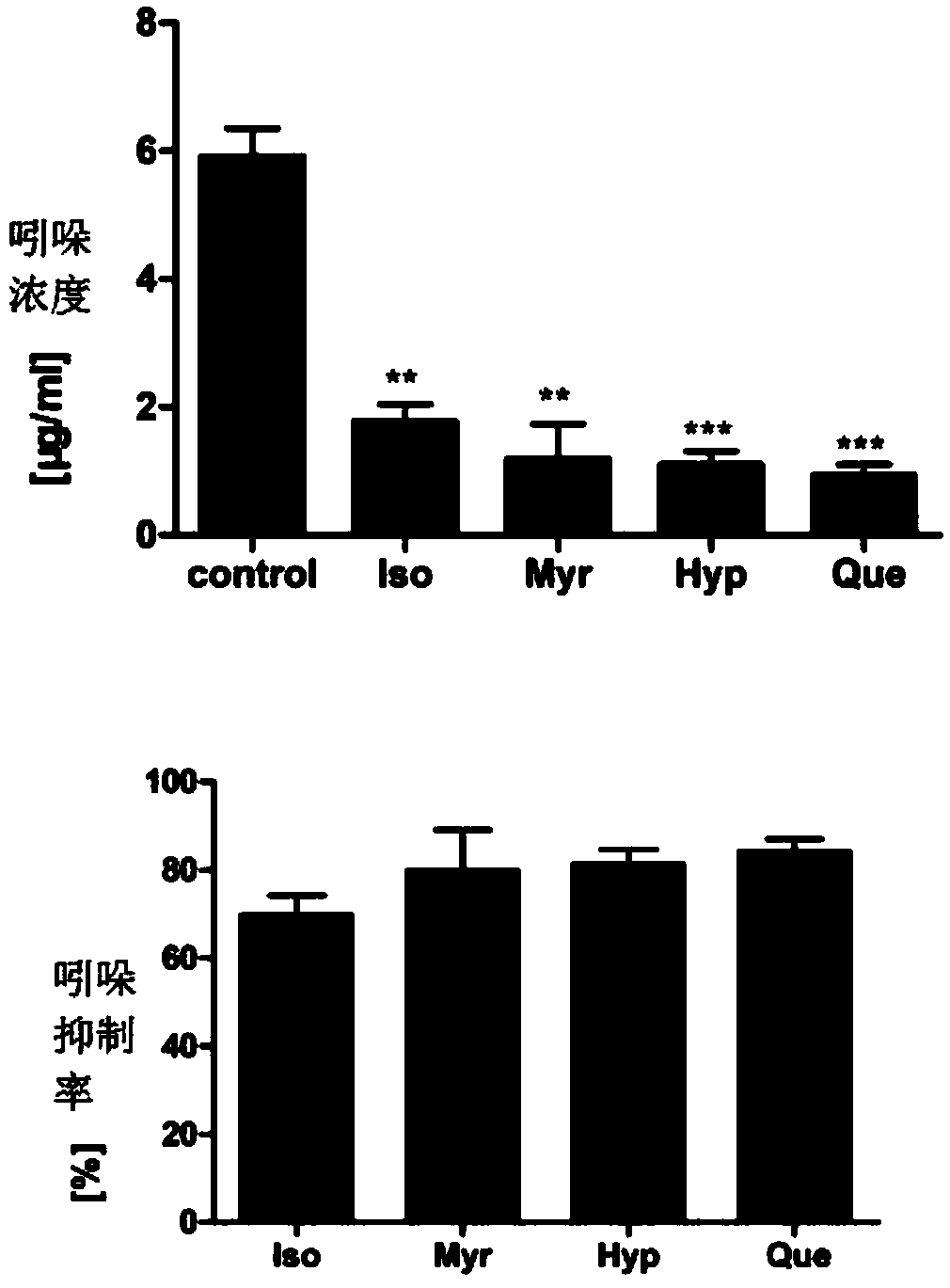
[0043] Example 3 Flavonoids reduce the synthesis of indole by mixed bacteria in rat feces
[0044] 1. Experimental method
[0045] With embodiment 1.
[0046] 2. Experimental results
[0047] Four compounds, isoquercitrin, myricetin, hyperin, and quercetin were selected, given a final concentration of 200 μM, and after anaerobic culture with rat fecal bacteria for 12 hours, the indole content in the bacterial supernatant was detected by HPLC. The inhibitory effects of the four compounds on indole secretion at a concentration of 200 μM were compared.
[0048] The results showed that the four compounds could inhibit bacterial secretion of indole to varying degrees (such as image 3 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com