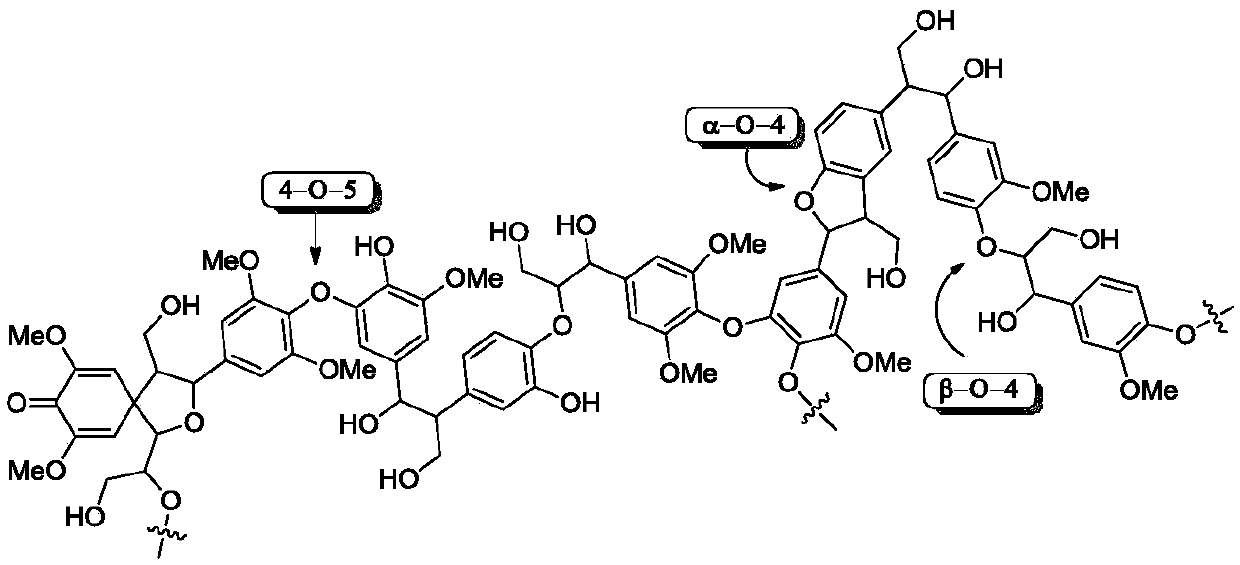
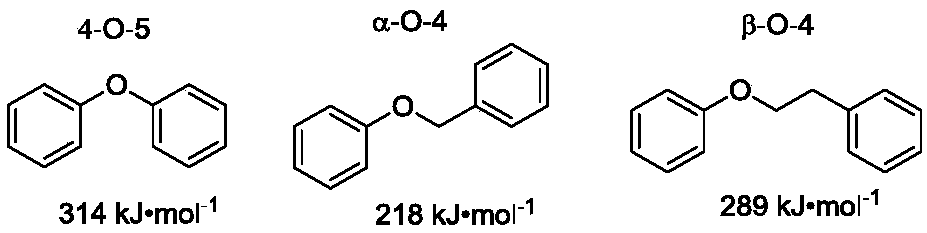
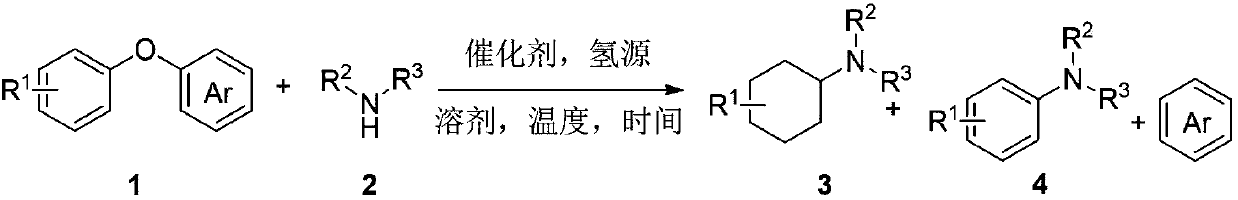
Synthesis method for converting lignin 4-O-5 model compound diaryl ether into nitrogen-containing compound
A 4-O-5, model compound technology, used in the preparation of amino compounds by condensation/addition reactions, organic chemistry, etc., can solve problems such as low industrial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Put a moderate stirring bar in a dry reaction tube (20 ml), add palladium hydroxide / carbon (30mol%), NaBH 4 (1.5 times the equivalent), then the reaction tube was evacuated, filled with argon, and replaced three times. Under the argon atmosphere, the solution of diphenyl ether (0.2 mmol) and hexahydropyridine (0.7 mmol) dissolved in the solvent was injected with a syringe Slowly add to the reaction tube, add 10 uL of water into the reaction tube with a micro-injector, then add 1 mL of air into the reaction tube with a syringe, heat the reaction tube in an oil bath at 160 degrees Celsius and stir the reaction. The reaction was stopped after 24 hours, the reaction tube was taken out from the oil bath, naturally cooled to room temperature, diluted with ethyl acetate, and then the reaction solution was filtered with diatomaceous earth, and the yield of benzene in the filtrate was 85% by gas phase detection. After the filtrate was concentrated, the final product...
Embodiment 2
[0028]
[0029] Put a moderate stirring bar in a dry reaction tube (20 ml), add palladium hydroxide / carbon (30mol%), NaBH 4 (1.5 times equivalent), then the reaction tube was evacuated, filled with argon, and replaced three times, and diphenyl ether (0.2mmol) and 2-methylpiperidine (0.7mmol) dissolved in the solvent were dissolved in the argon atmosphere. The solution was slowly added to the reaction tube with a syringe, 10 uL of water was added to the reaction tube with a micro injector, and 1 mL of air was added to the reaction tube with a syringe, and the reaction tube was heated in an oil bath at 160 degrees Celsius and stirred for reaction. The reaction was stopped after 24 hours, the reaction tube was taken out from the oil bath, naturally cooled to room temperature, diluted with ethyl acetate, and then the reaction solution was filtered with diatomaceous earth. The yield of benzene in the filtrate was 84% by gas phase detection. After the filtrate was concentrated...
Embodiment 3
[0031]
[0032] Put a moderate stirring bar in a dry reaction tube (20 ml), add palladium hydroxide / carbon (30mol%), NaBH 4 (1.5 times the equivalent), then the reaction tube was evacuated, filled with argon, and replaced three times, and the diphenyl ether (0.2mmol) and 3-methylpiperidine (0.7mmol) dissolved in the solvent were dissolved in the argon atmosphere. The solution was slowly added to the reaction tube with a syringe, 10 uL of water was added to the reaction tube with a micro injector, and 1 mL of air was added to the reaction tube with a syringe, and the reaction tube was heated in an oil bath at 160 degrees Celsius and stirred for reaction. After 24 hours, the reaction was stopped. The reaction tube was taken out from the oil bath, cooled to room temperature naturally, diluted with ethyl acetate, and then filtered with diatomaceous earth. The yield of benzene in the filtrate was 87% by gas phase detection. After the filtrate was concentrated, the final product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com