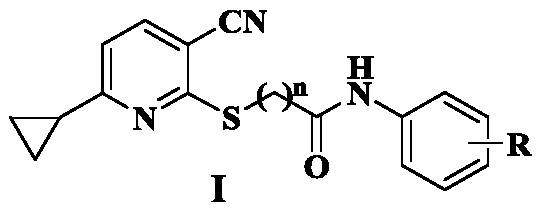
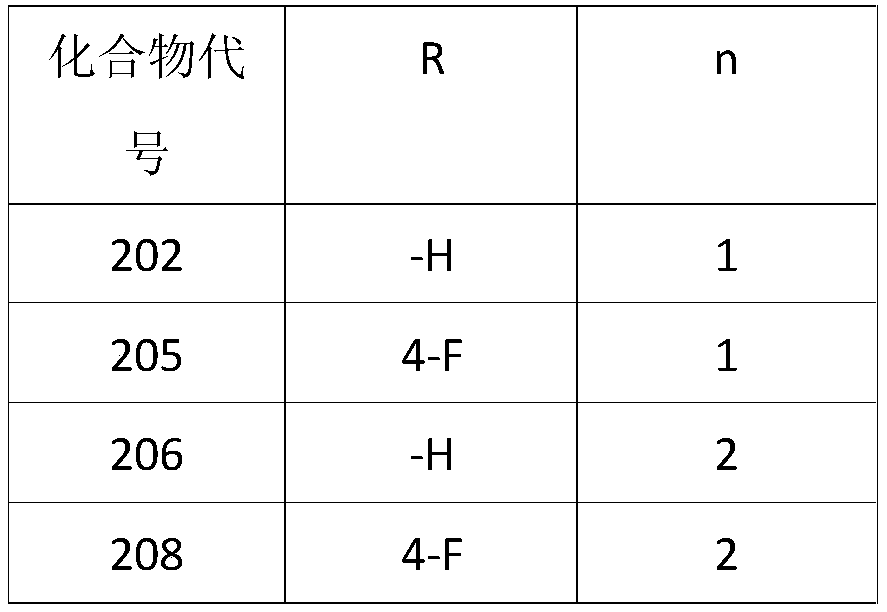
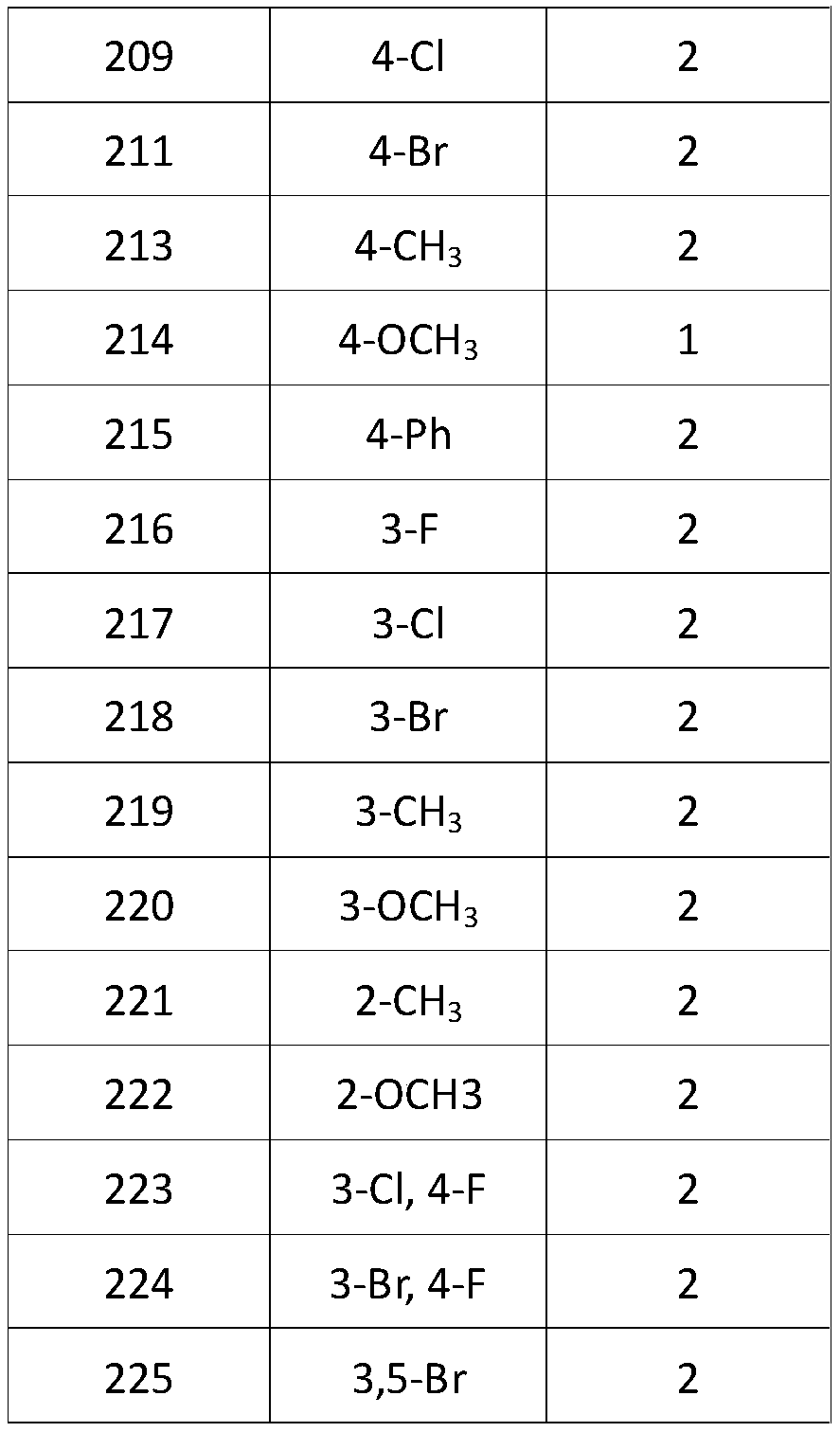
3-Cyano-6-cyclopropylpyridine type IDO1 inhibitor and application thereof
A methyl compound technology, applied in the field of medicinal chemistry, to achieve the effect of novel structure and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Preparation of Intermediate I-2
[0025]
[0026] In 30 mL of In 10% dilute sulfuric acid, stirred at room temperature for 30 min, slowly added an aqueous solution of ammonium persulfate (9.9 g, 43.4 mmol, 2 eq) dropwise, reacted overnight at room temperature, and TLC detected that the reaction was complete. Post-processing: adjust the pH to 8-9 with ammonia water, filter with suction, extract the filtrate with ethyl acetate three times, wash the organic phase with saturated brine once, evaporate the ethyl acetate under reduced pressure to obtain I-2 (3.5 g, harvested rate 90%).
[0027] Preparation of intermediate 1-3
[0028]
[0029] Dissolve thioglycolic acid (1.1g, 12mmol, 1.2eq) in DMSO, stir at room temperature for 10min, slowly add K 2 CO 3 (6.9g, 50mmol, 5eq) continued to stir at room temperature for 30min, then added dropwise the DMSO diluent of II-1 (1.8g, 10mmol, 1eq) and slowly warmed up to 60°C, reacted for 4h, and the reaction was complete by TL...
Embodiment 1
[0031] Preparation of 2-((3-cyano-6-cyclopropylpyridin-2-yl)thio)-N-phenylacetamide (1)
[0032] Dissolve II-2 (0.46g, 2mmol, 1eq), EDCI (0.50g, 2.6mmol, 1.3eq) and HOBT (0.08g, 0.6mmol, 0.3eq) in DMF, react at no more than 10°C for 30min, add After aniline (0.20g, 2.2mmol, 1.1eq), the color of the reaction solution became darker, and the reaction was carried out overnight at room temperature, and the reaction was complete as detected by TLC. Post-processing: Pour the reaction solution into water, filter with suction, and obtain the crude product by column chromatography on the filter cake, which is recrystallized with petroleum ether-ethyl acetate system to obtain 1 (140 mg, yield 57%). m.p.223-225℃.HRMS m / z[M+H] + calculated for C 17 h 15 N 3 OS:310.1009,found:310.1019. 1 H NMR (300MHz, DMSO-d 6 )δ:9.31(s,1H,-NH-),8.32(d,J=8.4Hz,1H,-ArH),7.67(d,J=8.0Hz,2H,-ArH),7.36-7.29(m, 5H,-ArH,-S-CH 2 -C=O-), 7.07(t, J=7.2Hz, 1H, -ArH), 2.25-2.19(m, 1H, -CH(CH 2 ) 2 -),1.07-1....
Embodiment 2
[0034] Preparation of Compound 2
[0035] Preparation of 2-((3-cyano-6-cyclopropylpyridin-2-yl)thio)-N-(4-fluorophenyl)acetamide
[0036]Dissolve I-3 (0.46g, 2mmol, 1eq), EDCI (0.50g, 2.6mmol, 1.3eq) and HOBT (0.08g, 0.6mmol, 0.3eq) in DMF, react at no more than 10°C for 30min, add After aniline (0.24g, 2.2mmol, 1.1eq), the color of the reaction solution became darker, and the reaction was carried out overnight at room temperature, and the reaction was complete as detected by TLC. Post-processing: Pour the reaction solution into water, filter with suction, and obtain a crude product by column chromatography of the filter cake, which is recrystallized with petroleum ether-ethyl acetate system to obtain 2 (160 mg, yield 61%). m.p.198-200℃.HRMS m / z[M+H] + calculated for C 17 h 14 FN 3 OS:328.0914,found:328.0926. 1 H NMR (300MHz, DMSO-d 6 )δ:9.42(s,1H,-NH-),8.33(d,J=8.4Hz,1H,-ArH),7.69(dd,J=7.9,5.1Hz,2H,-ArH),7.36(m, 3H,-ArH,-S-CH 2 -C=O-), 7.16(t, J=8.9Hz, 2H, -ArH), 2.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com