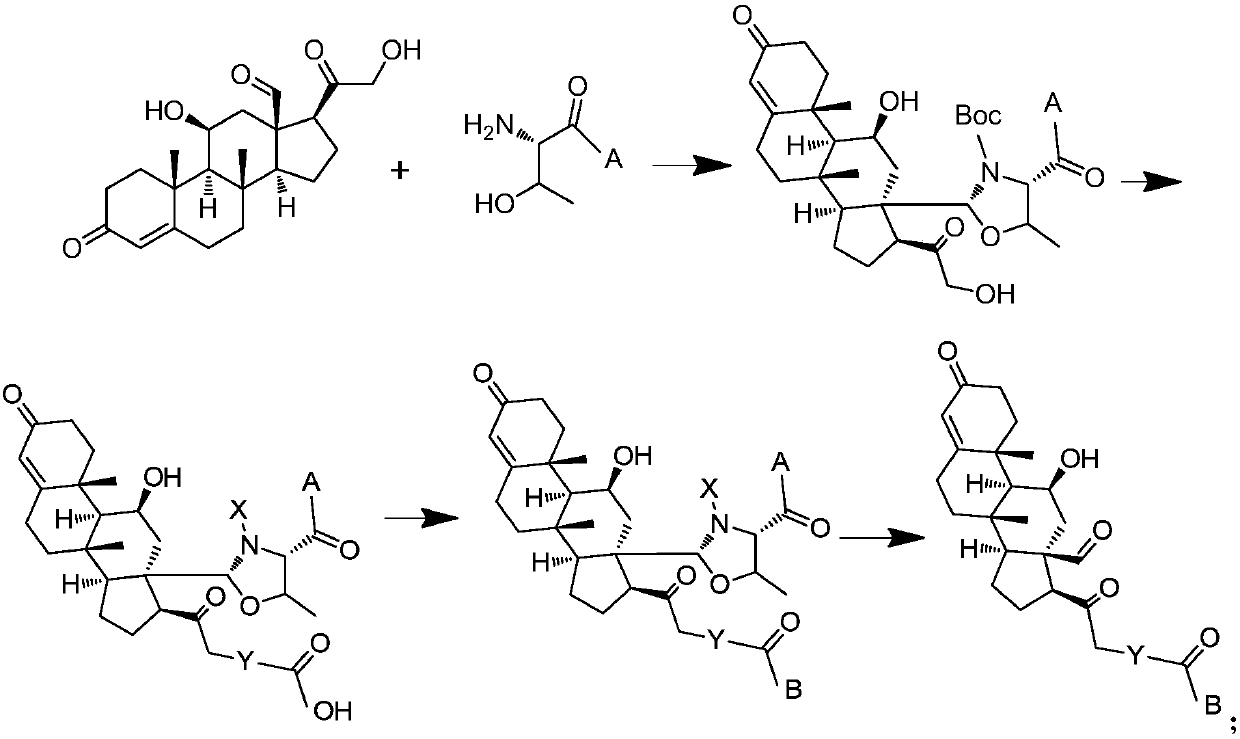
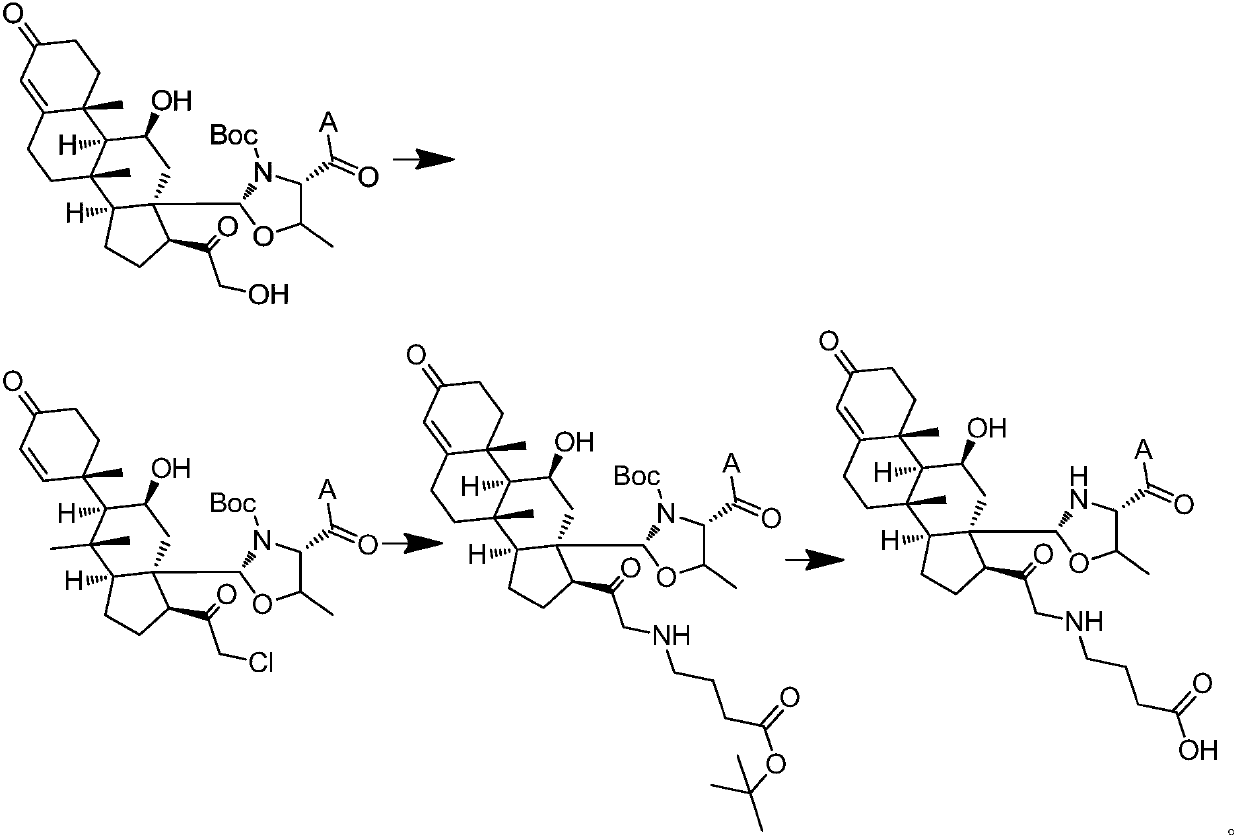
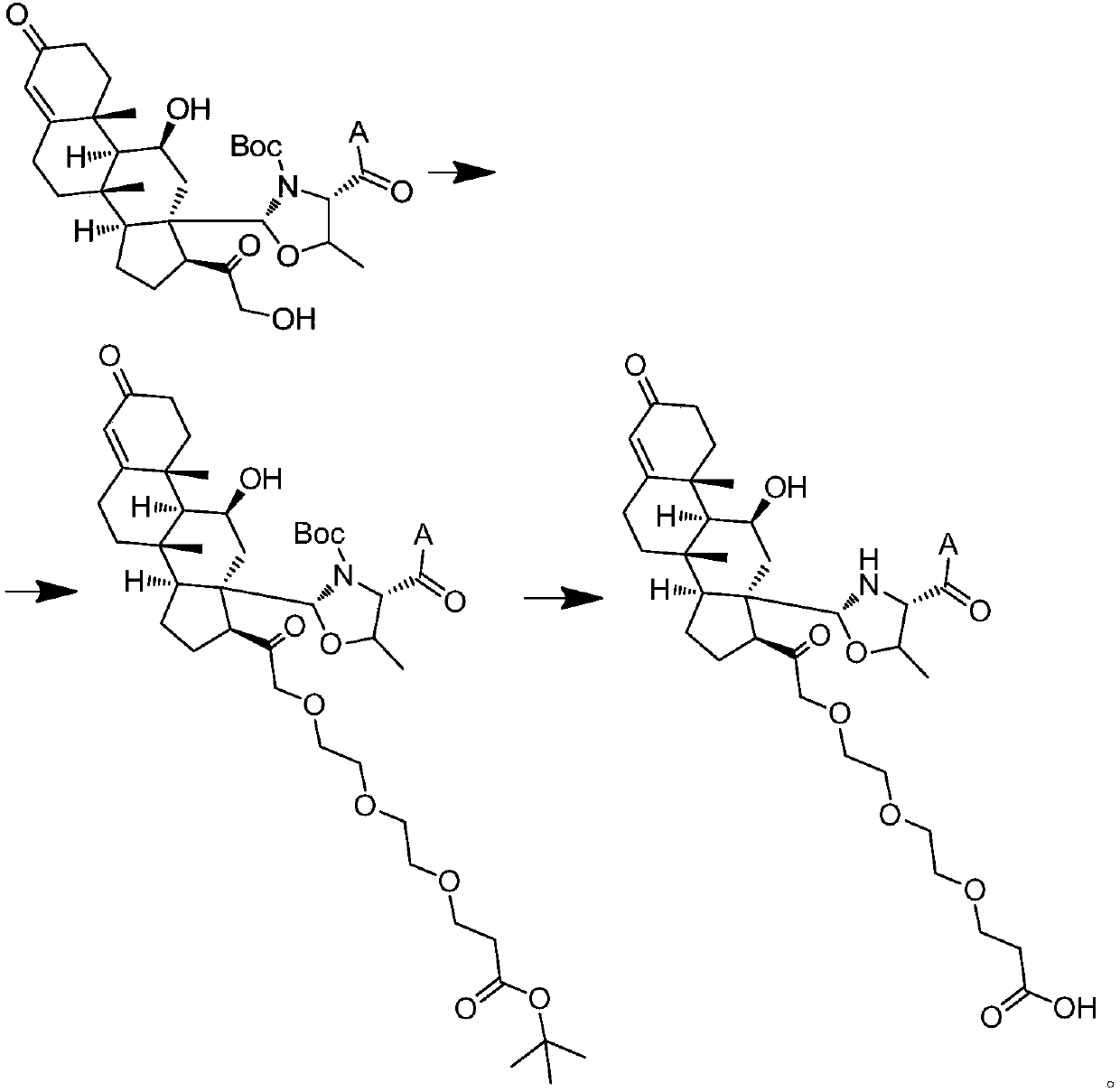
Aldosterone derivative and preparation method thereof
An aldosterone and derivative technology, applied in the field of aldosterone derivatives and their preparation, can solve the problems of complete antigen stability and homogeneity, complete antigen specificity and poor immunogenicity, difficult to form site-specific cross-linking, etc., and achieve linear improvement. , Overcome differences in affinity, and promote the effect of popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Synthesis of MBHA resin with specific functional groups
[0100] 1. Weigh 100mg of MBHA resin (1) (sigma, 200-400 mesh, 0.5-1.0mmol / g), add 10ml of DMF (Chinese medicine) to dissolve, blow nitrogen or argon into the solution, and control the flow rate of the resin by adjusting the airflow Mixing degree, add 0.3mmol Fmoc-Thr-OH (Shanghai Jill), 0.6mmol EDC.HCl (sigma), 0.6mmol HOBT (Shanghai Jill), 1.8mmol DIPEA (Sinopharm), mix well, and react at room temperature for 1h.
[0101] 2. Stop the reaction, remove the reaction solution by filtration, wash twice with DMF, add 5ml DMF, 0.1mmol acetic anhydride and 0.3mmol DIPEA, and react at room temperature for 10min.
[0102] 3. Stop the reaction, remove the reaction solution by filtration, wash twice with DMF, add 10ml of 20% piperidine / DMF, and react at room temperature for 30min.
[0103] 4. Stop the reaction, remove the reaction solution by filtration, and wash twice with DMF to obtain MBHA resin (2) with specific functi...
Embodiment 2
[0107] Aldosterone Conjugated MBHA Resin
[0108] 1. Configure 10ml of a mixed solution of MeOH:DCM:DMF:AcOH=30:3:2:0.35, add to the MBHA resin (2) with specific functional groups obtained in the above steps, blow nitrogen or argon into the reaction solution, and pass Adjust the air flow to control the mixing degree of the resin, add 0.3mmol aldosterone, and react at room temperature.
[0109] 2. Stop the reaction, remove the reaction solution by filtration, wash twice with DCM, add 10ml DCM, 0.6mmol Boc2O and 0.9mmol DIPEA, and react at room temperature for 3h.
[0110] 3. Stop the reaction, remove the reaction solution by filtration, wash it twice with DCM, then filter it with suction for 1-2 hours until the resin is completely dry, weigh the mass to get 240 mg, and calculate according to the theoretical amount, 80 mg of MBHA resin (4) is about conjugated with 0.1 mmol aldosterone.
[0111] The synthetic route is as follows:
[0112]
Embodiment 3
[0114] Introducing the cross-link arm
[0115] 1. Introduction of succinic anhydride cross-linking arms
[0116] Weigh 80 mg of MBHA resin (4) that has been conjugated with aldosterone, dissolve it in 5 ml of DMF, blow nitrogen or argon into the reaction solution, and control the mixing degree of the resin by adjusting the air flow, add 0.3 mmol of succinic anhydride (Aladdin), 0.1mmol DMAP (Sinopharm), react at room temperature for 3h. Stop the reaction, remove the reaction solution by filtration, wash twice with DMF, and filter with suction for 1-2 hours until the resin is completely dry, then store it for use.
[0117] The reaction scheme is as follows:
[0118]
[0119] 2. Introduce γ-aminobutyric acid cross-linking arm
[0120] 1) Weigh 80 mg of MBHA resin (4) that has been conjugated with aldosterone, dissolve it in 5 ml of DMF, blow nitrogen or argon into the reaction solution, and control the mixing degree of the resin by adjusting the air flow, add 0.15 mmol met...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com