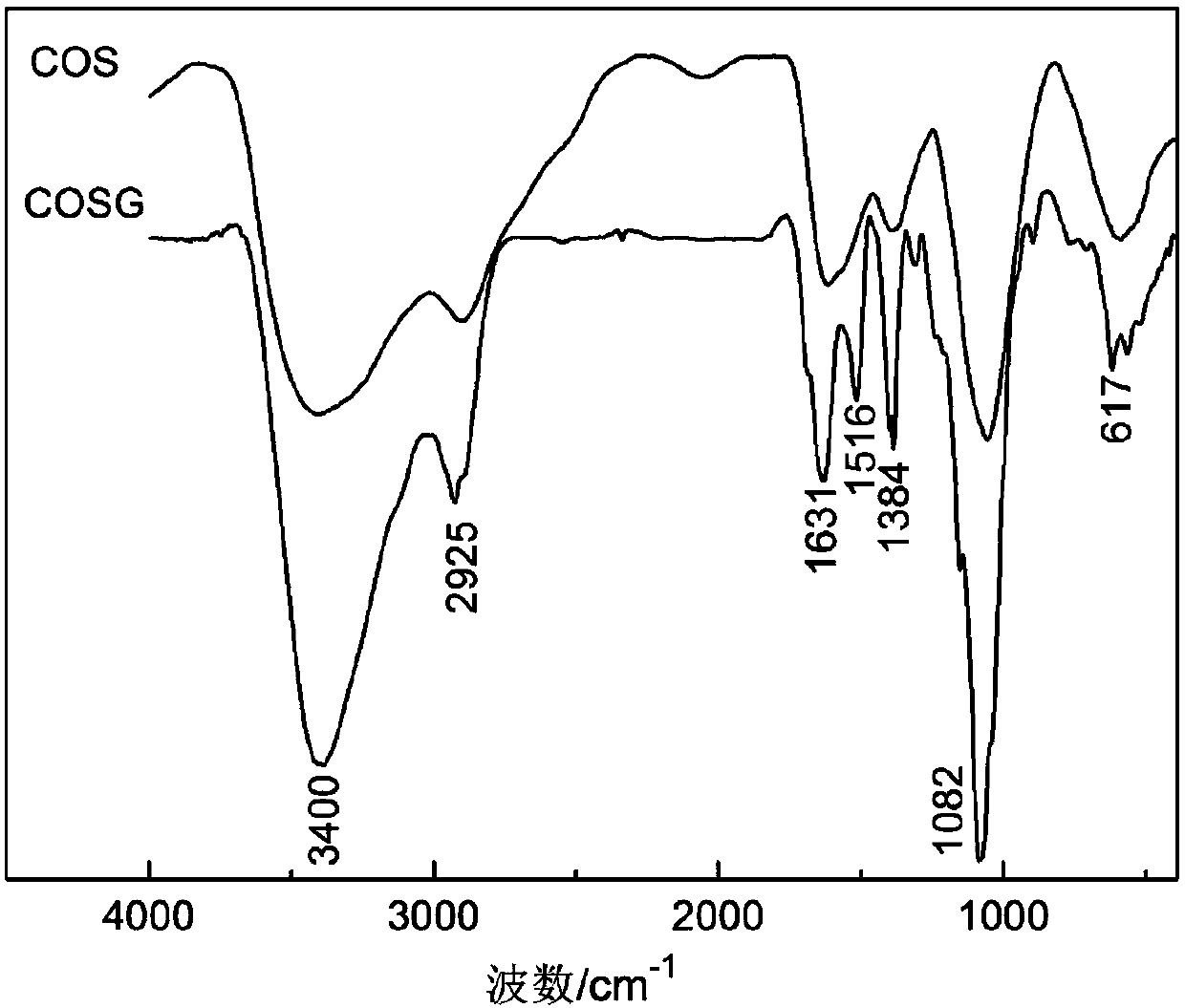
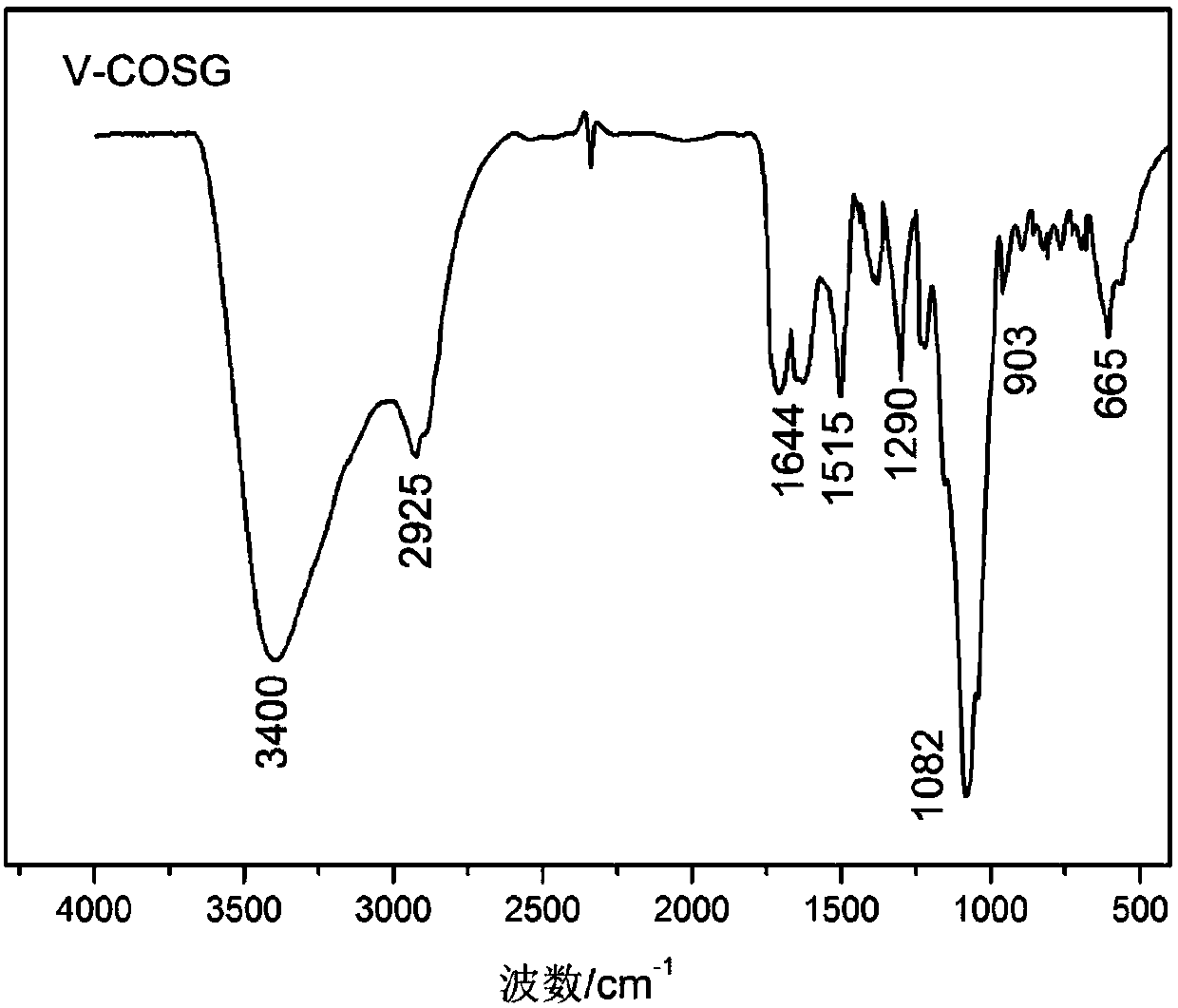
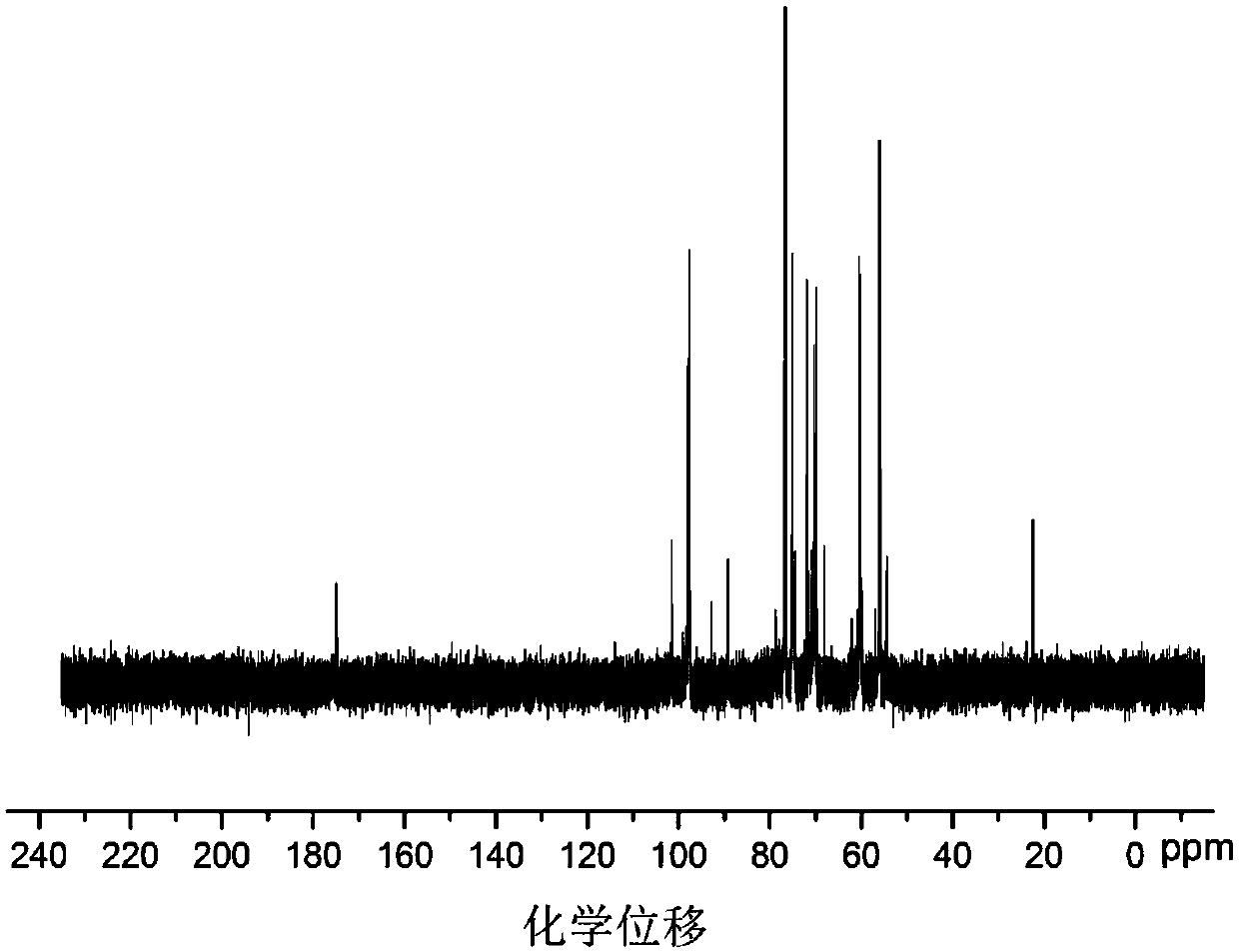
Application of vanillin crosslinked chitosan oligosaccharide mono guanidine hydrochloride in preparation of anti-oxidation drugs
A technology of chitosan oligosaccharide and vanillin, which is applied in the directions of drug combination, antidote, pharmaceutical formulation, etc., can solve the problems of loose material structure, toxicity, poor controlled release effect, etc., and achieves high microwave efficiency, short reaction time, improved The effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] a. Synthesis of thiourea trioxide: mix hydrogen peroxide solution with concentrated sulfuric acid solution, stir at 40~80℃ for 10~30min, then alternately add thiourea dioxide solid and hydrogen peroxide sulfuric acid mixed solution, within 3~7h After the feeding is completed, the temperature is kept and stirred for 1 to 3 hours to generate a large number of granular white crystals and then the reaction is stopped. After the reaction solution is cooled to room temperature, precipitation, suction filtration and drying are carried out to obtain white crystals of thiourea trioxide;
[0036] b. Synthesis of Chitooligosaccharide Monoguanidine Hydrochloride:
[0037] (1) Weigh 2g of chitosan oligosaccharides dissolved in 0.8mol / L hydrochloric acid solution to prepare chitosan oligosaccharide hydrochloric acid solution, adjust the pH to 1, and then weigh 0.5 times amino mole of thiourea trioxide and dissolve it in distilled water. For thiourea trioxide solution, add thiourea trioxid...
Embodiment 2
[0042] a. Synthesis of thiourea trioxide: mix hydrogen peroxide solution with concentrated sulfuric acid solution, stir at 40~80℃ for 10~30min, then alternately add thiourea dioxide solid and hydrogen peroxide sulfuric acid mixed solution, within 3~7h After the feeding is completed, the temperature is kept and stirred for 1 to 3 hours to generate a large number of granular white crystals and then the reaction is stopped. After the reaction solution is cooled to room temperature, precipitation, suction filtration and drying are carried out to obtain white crystals of thiourea trioxide;
[0043] b. Synthesis of Chitooligosaccharide Monoguanidine Hydrochloride:
[0044] (1) Weigh 2g of chitosan oligosaccharides dissolved in 0.2mol / L hydrochloric acid solution to prepare chitosan oligosaccharides hydrochloric acid solution, adjust the pH to 1 and then weigh 2 times the amino mole of thiourea trioxide and dissolve in distilled water to form three To oxidize thiourea aqueous solution, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com