A kind of leucine-5-hydroxylase mutant and its application
A leucine and mutant technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of many by-products, complex process routes, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
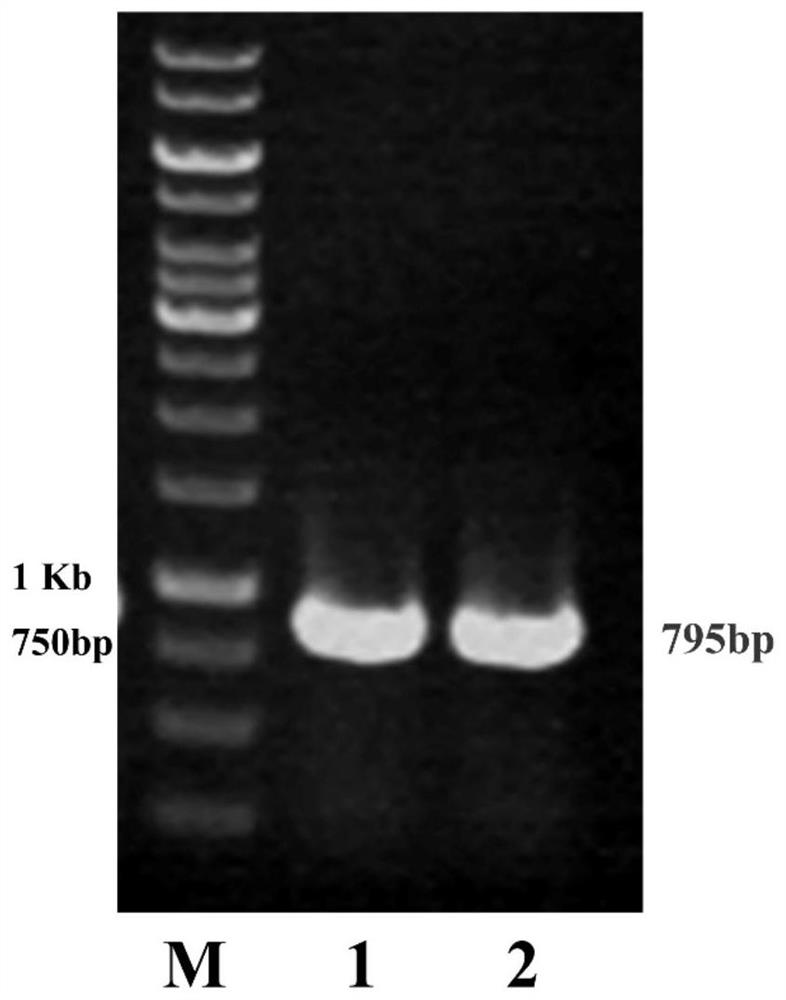
[0050] Example 1. Obtaining of the gene mLEH encoding the leucine-5-hydroxylase mutant V77A
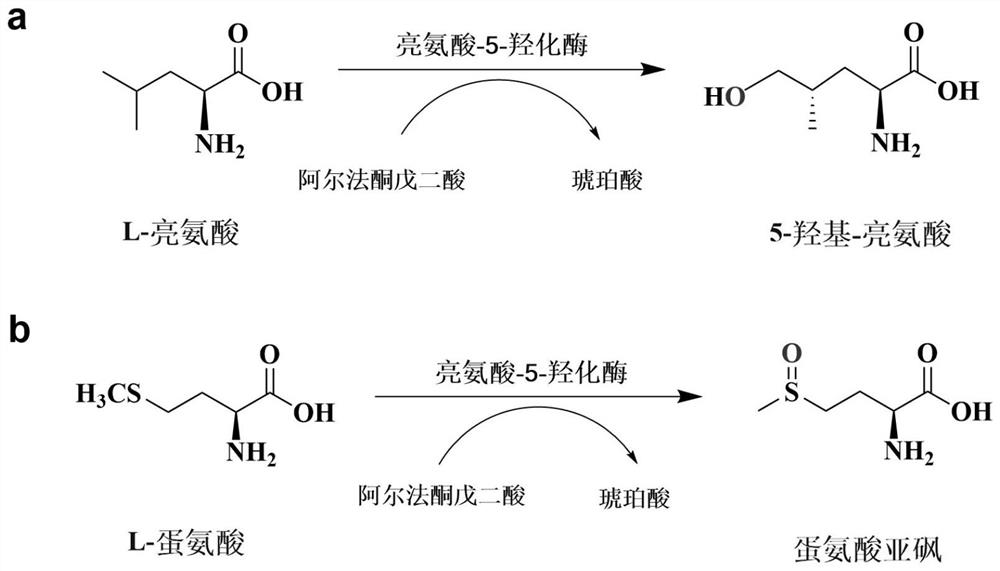
[0051] Obtaining mutant V77A: the gene of wild-type leucine-5-hydroxylase derived from Nostoc sp. was codon-optimized (SEQ ID NO.3) and constructed in pET28a(+) expression vector (wild-type leucine- Genbank sequence number RCJ32143.1 of 5-hydroxylase) to obtain LEH-pET28a; using LEH-pET28a plasmid as template, V77A_F (SEQ ID NO.5), V77A_R (SEQ ID NO.6) as primers, reverse PCR site-directed mutagenesis; the experiment in this example uses the KOD-Plus mutant kit (purchased from Toyobo (Shanghai) Biotechnology Co., Ltd.):
[0052] (1) The PCR reaction system is as follows:
[0053] Template (53ng / μL) 1μL Primer F (10pmol / μL) 1.5μL Primer R (10pmol / μL) 1.5μL 2mM dNTPs 5μL 10×Buffer for iPCR 5μL KOD-Plus 1μL wxya 2 o
35μL
[0054] PCR reaction conditions: pre-denaturation at 94°C for 2min; denaturation at 98°C for 10sec; exte...
Embodiment 2
[0061] Embodiment 2, expression of leucine-5-hydroxylase
[0062] The recombinant vectors LEH-pET28a and mLEH-pET28a obtained by constructing the wild-type coding gene LEH and the mutant coding gene mLEH respectively on the pET28a(+) expression vector were transformed into Escherichia coli BL21(DE3), and BL21 / LEH and BL21 / V77A recombinant bacteria.
[0063] The above two recombinant bacteria were respectively inoculated in 5 mL LB medium (containing 50 μg / mL kanamycin), and cultured at 220 r / min at 37 ° C for 12 h; Kanamycin), to be bacteria concentration OD 600 =0.6-0.8, add IPTG, the final concentration is 0.75mM, induce at 16°C, 180r / min for 20h.
Embodiment 3
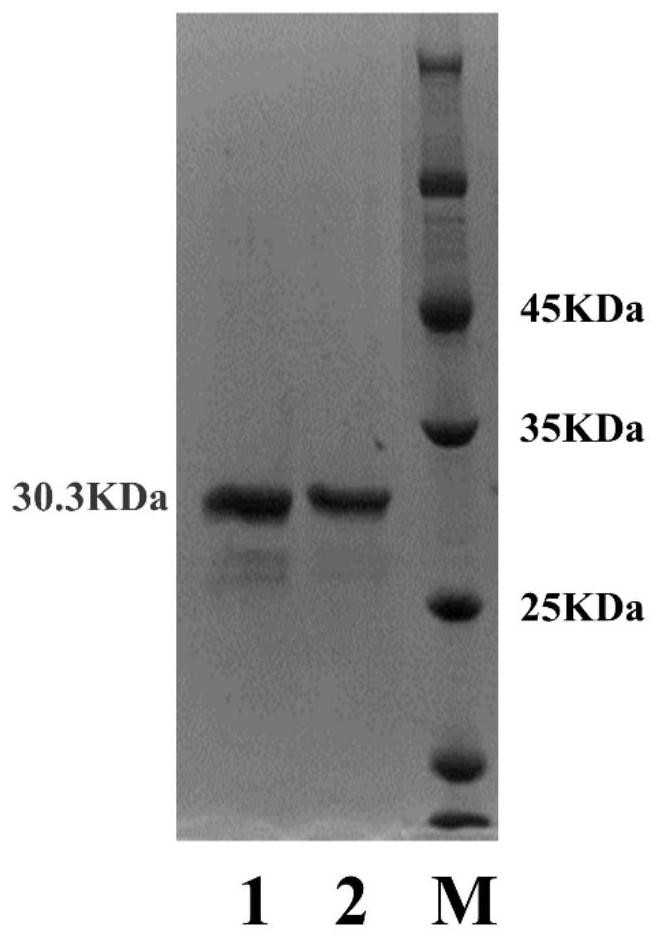
[0064] Example 3, Purification and Refining of Leucine-5-Hydroxylase
[0065] The bacterium solution obtained in Example 2 was centrifuged at 5000r / min and 15min to collect the thalline, and after resuspending with solution A (20mM Tris-HCl, pH 8.0, 300mM NaCl, 20mM imidazole, 1.5mM DTT), add lysozyme ( The final solubility is 200μg / mL), protease inhibitor (final solubility is 1mM) placed on ice for 30min, sonicated on ice (3s on, 5s off, 350W power), low temperature and high-speed centrifugation (4°C, 18000r / min) Cell debris was removed to obtain the supernatant.
[0066] Ni affinity chromatography: take 4 open columns (Open-Column), and add 1 mL Ni-NTA resin (QIAGEN) to each. Equilibrate the resin with 20 mL of Solution A. The high-speed centrifuged supernatant was combined with 1 mL of resin at 4°C for 40-60 min. Pass the mixture through an open column, and the resin bound to the protein will be retained. Rinse the resin with 20 mL of solution A. Finally, the protein w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com