Positive electrode material preparation used for lithium ion batteries and modification method thereof
A lithium-ion battery and cathode material technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem of low discharge capacity, achieve the effects of reducing manganese dissolution, increasing capacity, and stabilizing the lattice structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
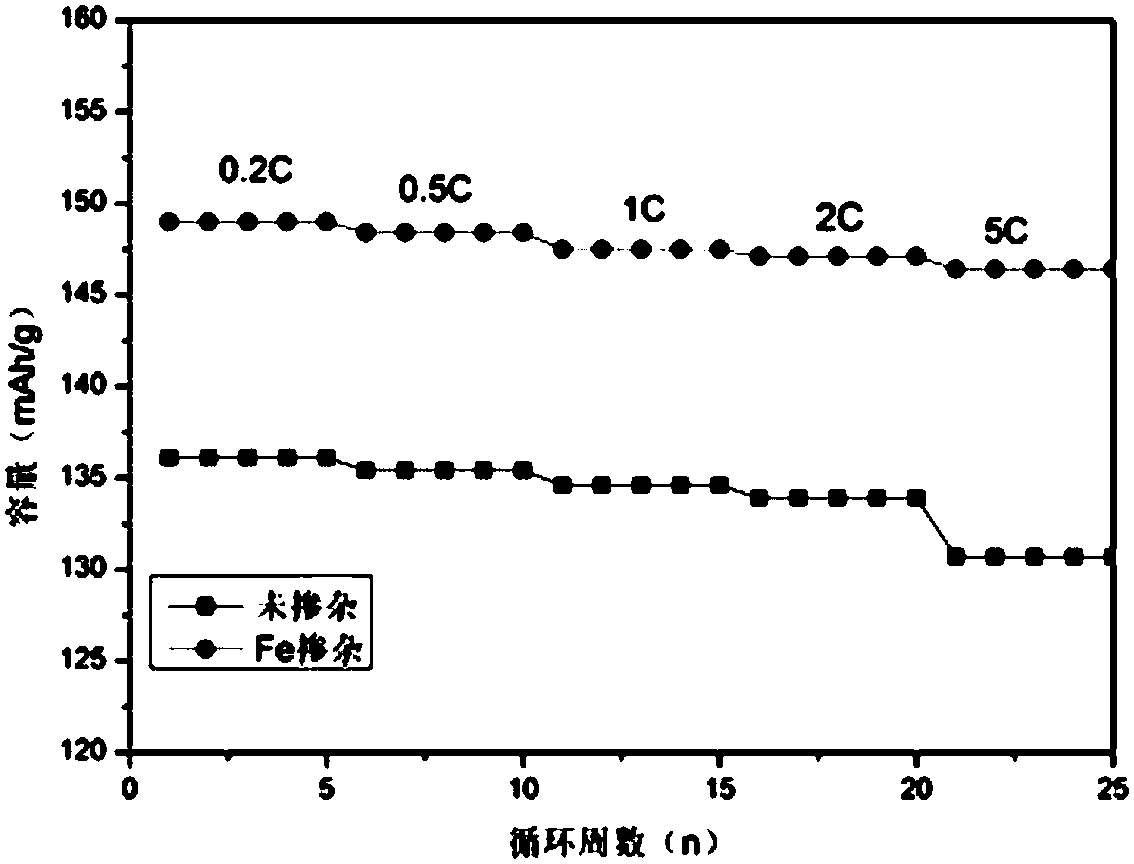
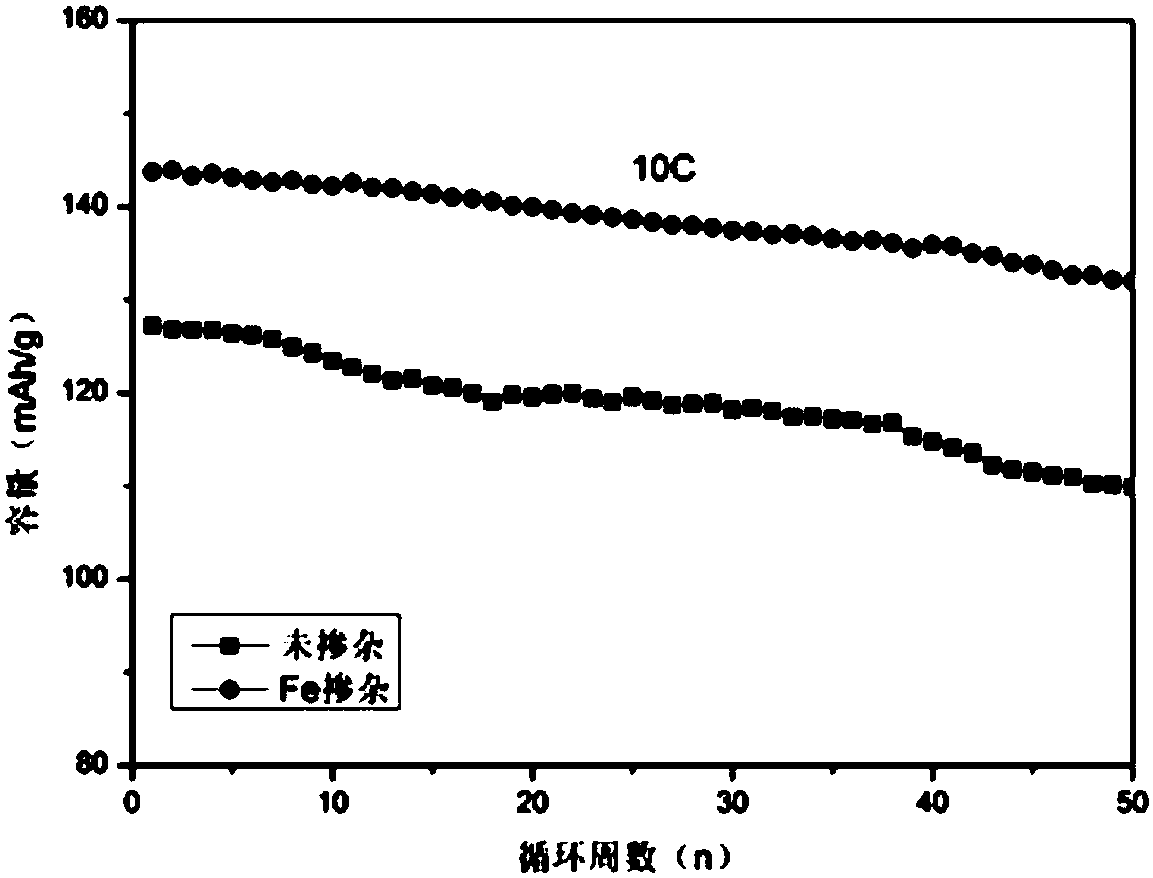
[0027] Fe-doped nickel manganese oxide material LiNi 0.5-x Mmm 1.5-x Fe 2x 0 4 (x=0.05) Preparation
[0028] 1. Mix 0.268g LiCH 3 COO·2H 2 O, 0.280g Ni(CHsCOO) 2H 2 O, 0.888g Mn(CH 3 COO)·4H 2 O, 0.0615g Fe(CH 3 COO)2 4H 2 O was dissolved in 20 mL of deionized water, and 80 mL of ethanol was added for stirring to obtain a metal salt solution.
[0029] 2. Add 0.780g of H 2 C 2 o 4 Dissolve in 30mL deionized water to form an oxalic acid solution.
[0030] 3. Quickly pour the metal salt solution into the oxalic acid solution and stir for 5 hours, and then evaporate at 80° C. for 10 hours to obtain the precipitation of the oxalate precursor.
[0031] 4. Raise the temperature at a rate of 5C / min, pre-calcine the oxalate precursor in the air at 450°C for 8 hours, and then calcinate at the same rate to 800°C for 15 hours to obtain the target product LiNi 0.45 mn 1.45 Fe 0.1 o 4 .
[0032] The prepared Fe-doped lithium nickel manganese oxide material LiNi 0.5-x Mmm...
Embodiment 2
[0034] Co-doped nickel-rich layered Li-ion battery cathode material Li 1.2 Ni 0.19 co 0.01 mn 0.6 o 2 preparation of
[0035] 1. Add 0.402g LiCH 3 COO·2H 2 O, 0.149g Ni(CHsCOO) 2 2H 2 O, 0.460g Mn(CHsCOO) 2 4H 2 O, 0.050g Co(CH 3 COO) 2 4H 2 O was dissolved in 20 mL of deionized water, and then 80 mL of ethanol was added for stirring to obtain a metal salt solution.
[0036] 2. Add 0.570g of H 2 C 2 o 4 Dissolve in 10mL deionized water and 40ml ethanol to form an oxalic acid solution.
[0037] 3. Quickly pour the metal salt solution into the oxalic acid solution and stir for 5 hours, and then evaporate at 80° C. for 10 hours to obtain the precipitation of the oxalate precursor.
[0038] 4. Raise the temperature at a rate of 5°C / min, pre-calcine the oxalate precursor in the air at 450°C for 8 hours, and then raise the temperature to 850°C for 20 hours at the same heating rate to obtain the target product Li 1.2 Ni 0.19 co 0.01 mn 0.6 o 2 .
[0039] The pr...
Embodiment 3
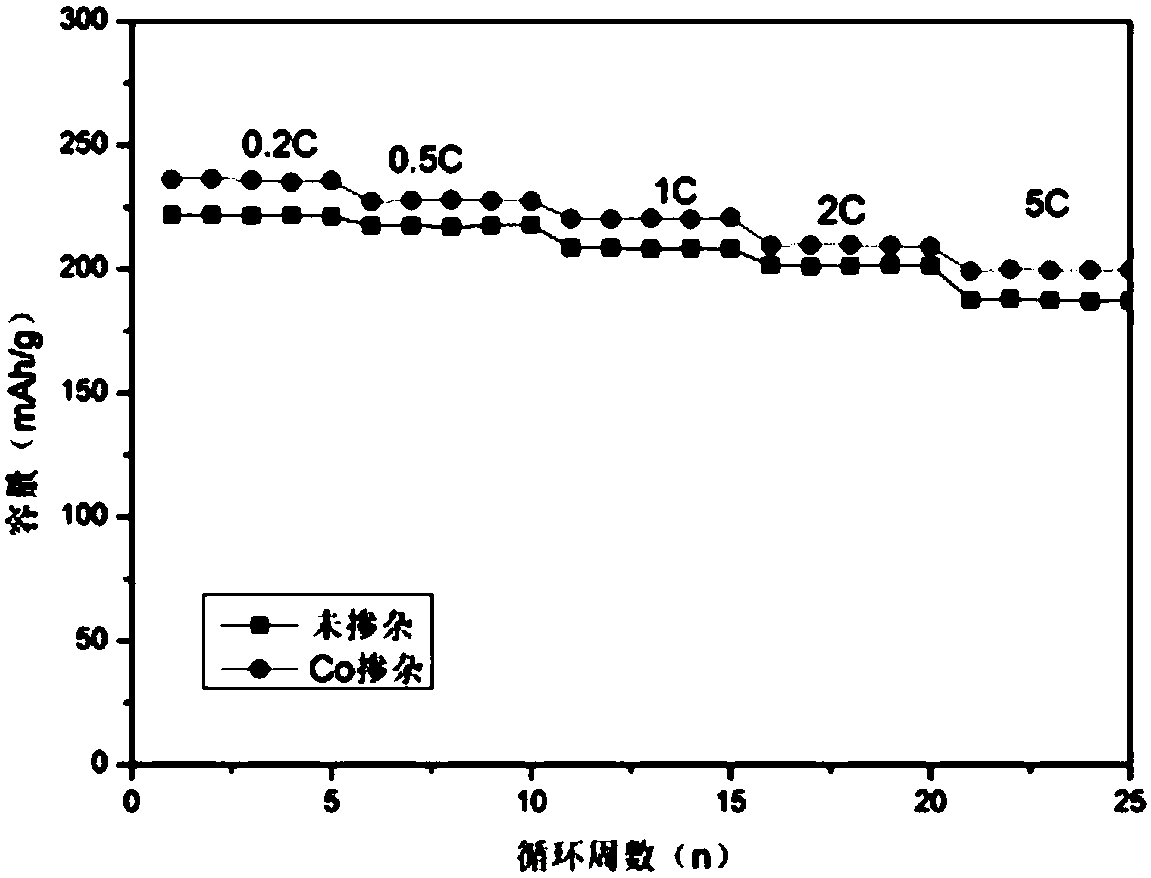
[0041] Cr, Co co-doped lithium nickel manganese oxide material LiNi 0.45 mn 1.45 co 0.06 Cr 0.05 o 4 preparation of
[0042] 1. Add 0.268gLiCH 3 COO·2H 2 O, 0.280gNi(CH 3 COO) 2 2H 2 O, 0.888g Mn(CH 3 COO) 2 4H 2 O, 0.031g Co(CH 3 COO) z 4H 2 O, 0.029g Cr(CCH 3 COO) 2 Dissolve in 20mL of deionized water, then add 80mL of ethanol and stir to obtain a metal salt solution.
[0043] 2. Add 0.78g of H 2 C 2 o 4 Dissolve in 40mL deionized water to obtain oxalic acid solution.
[0044] 3. Quickly pour the metal salt solution into the oxalic acid solution and stir for 5 hours, and then evaporate at 80° C. for 10 hours to obtain the precipitation of the oxalate precursor.
[0045] 4. Raise the temperature at a rate of 5°C / min, deposit the oxalate precursor in the air at 450°C for 8 hours, and then calcinate at 850°C for 20 hours at the same heating rate to obtain the target product LiNi 0.45 mn 1.45 co 0.06 Cr 0.05 o 4 .
[0046] The prepared Co, Cr co-doped ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com