Intradermal injection technology for enhancing viral antigen immunogenicity and application of intradermal injection technology in research of hand-foot-mouth disease vaccine
A technology for hand, foot and mouth disease and vaccines, applied in the direction of virus antigen components, vaccines, viruses, etc., can solve the problems of complex etiology, limited cross-protection ability, etc., and achieve good growth and replication, good safety, and improved quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The present embodiment provides a kind of preparation method of bivalent hand, foot and mouth disease inactivated vaccine for human use, and the specific steps are as follows:
[0042] (1) Cell culture: According to the KMB-17 cell production and verification regulations, select the 28th passage KMB-17 cells with dense growth, discard the culture medium, digest with 0.1% trypsin, discard the trypsin solution, and use the original culture medium volume 4% volume of DMEM to suspend the cells to obtain a cell suspension;
[0043] (2) Virus inoculation and culture: Add an appropriate amount of the above cell suspension into the cell factory, mix well and place at 37°C for 3-4 days; after growing into a dense monolayer, discard the culture medium; add EVA71 or CVA16 virus seeds respectively , the moi of the inoculum is 0.02-0.05; after mixing, place it at 37°C and shake it gently at the right time; after 30 minutes, add quantitative cell growth medium and place it at 37°C fo...
Embodiment 2
[0049] This example is the distribution detection of various intermediate products during the preparation process of the human bivalent inactivated hand, foot and mouth disease vaccine described in Example 1.
[0050] Among them, the virus harvest liquid, virus concentrated liquid, inactivated and purified antigen liquid, and semi-finished products are all clear liquids without foreign matter and precipitation. The sterility test and mycoplasma test are all negative, and there is no abnormal toxin, pH 7.2-8.0, see Table 1 .
[0051]
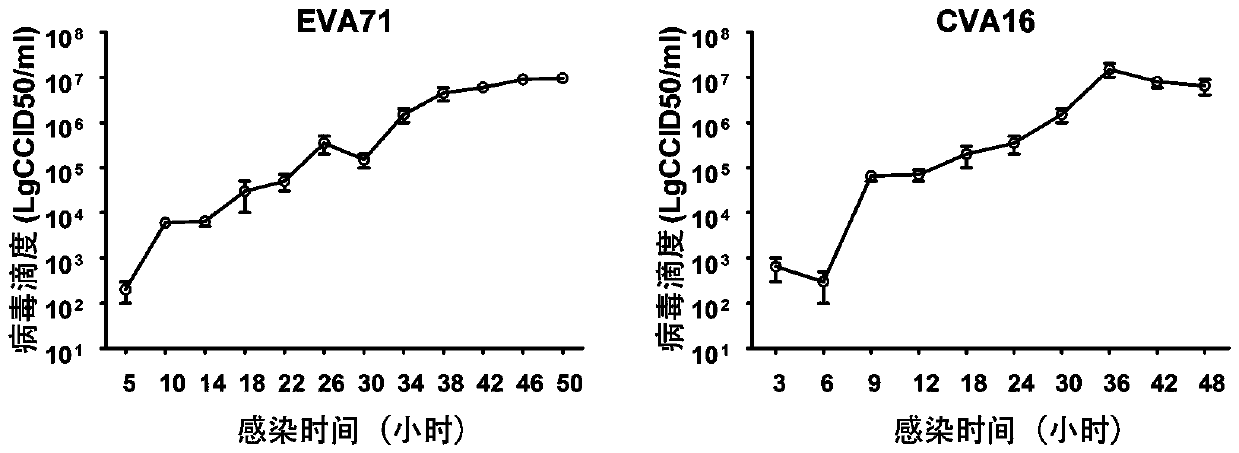
[0052] Such as figure 1 As shown, samples were taken at different time points after the virus was inoculated into KMB17 cells, and the infectious titer was detected. The results show that initial typical lesions can appear within 8 hours of inoculating cells, and the peak of lesions can appear within 24-48 hours, and the titer is usually 7.0±0.5LogCCID 50 .
Embodiment 3
[0054] This example tests the safety of the finished product of the bivalent inactivated vaccine for human use prepared in Example 1.
[0055] The finished product of the vaccine was injected at 0.2 mL / only into the abdominal cavity, intradermal, and muscle of mice with a body weight of 18-20 g. There were 20 mice in each group. There should be no death within one month. The weight gain of the mice was the same as that of the normal saline control group, see Table 2.
[0056]
[0057] The formaldehyde content of the finished vaccine is not higher than 50μg / dose, and the bacterial endotoxin is not higher than 100EU / dose. And it was negative in the sterility test, see Table 3.
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com