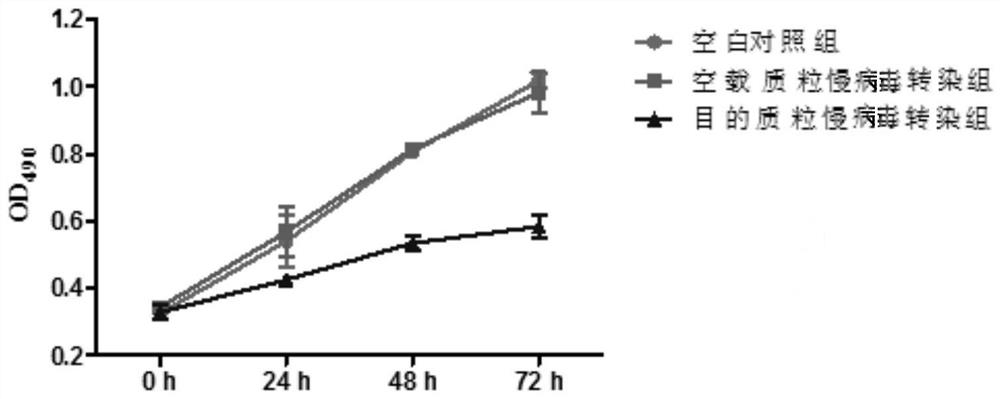
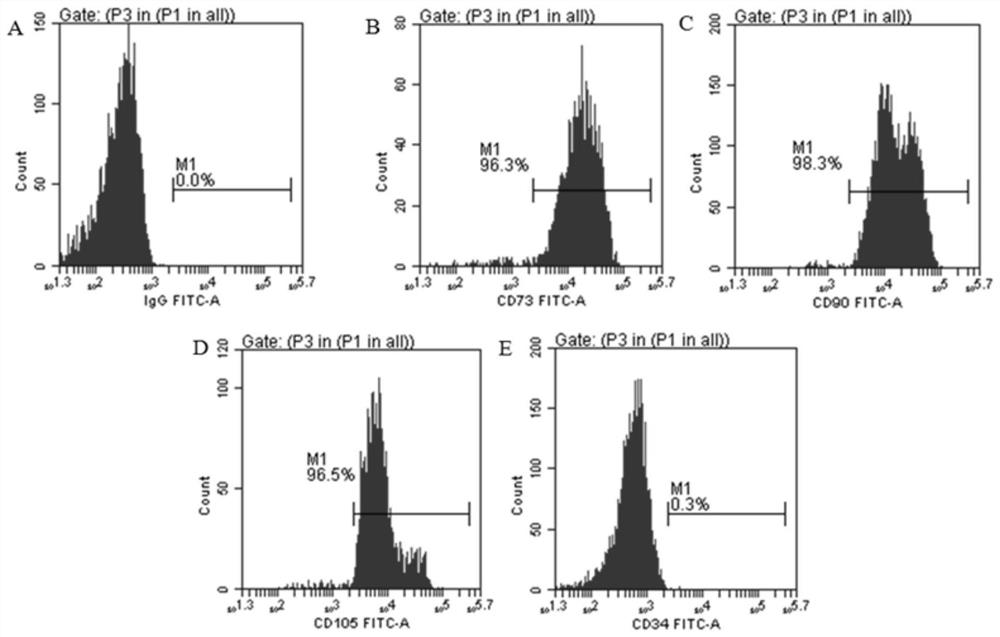
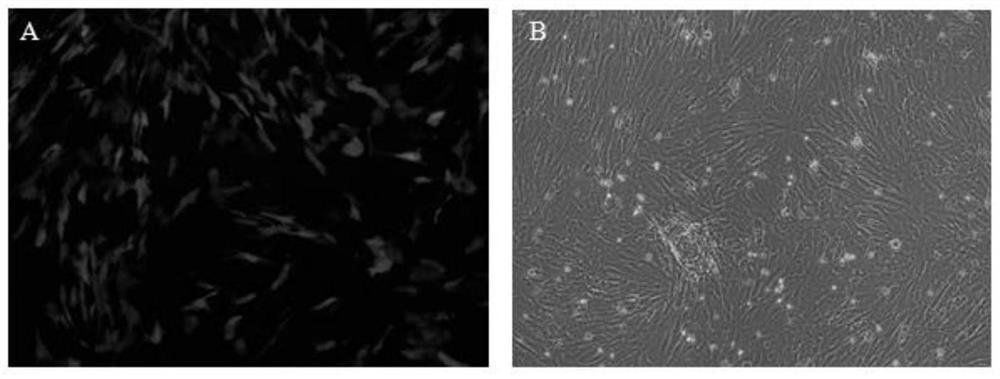
Umbilical cord mesenchymal stem cells silenced by mir338 gene and its preparation method and application
A technology of mesenchymal stem cells and gene silencing, applied in the fields of genetic engineering and biomedical engineering, can solve problems such as complex pathophysiological mechanisms and unclear understanding, and achieve good therapeutic effects, good clinical application prospects, and accelerated cell proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Construction and identification of lentiviral particles overexpressing mir-338
[0036] 1. Obtaining the target plasmid
[0037] The lentiviral system vector pLVX-DsRed-Monomer-N1 was used, and the cDNA sequence of the target gene was ligated to the vector, and the restriction site was AsuII / BamHI, and the target plasmid mir-338p-pLVX-DsRed-Monomer was obtained by gene synthesis -N1, the sequence of the recombinant plasmid mir-338p-pLVX-DsRed-Monomer-N1 is shown in SEQ ID NO:1.
[0038] SEQ ID NO: 1
[0039]TGGAAGGGCTAATTCACTCCCAAAGAAGACAAGATATCCTTGATCTGTGGATCTACCACACACAAGGCTACTTCCCTGATTAGC AGAACTACACACCAGGGCCAGGGGTCAGATATCCACTGACCTTTGGATGGTGCTACAAGCTAGTACCAGTTGAGCCAGATAAGGTAGA AGAGGCCAATAAAGGAGAGAACACCAGCTTGTTACACCCTGTGAGCCTGCATGGGATGGATGACCCGGAGAGAGAAGTGTTAGAGTGG AGGTTTGACAGCCGCCTAGCATTTCATCACGTGGCCCGAGAGCTGCATCCGGAGTACTTCAAGAACTGCTGATATCGAGCTTGCTACA AGGGACTTTCCGCTGGGGACTTTCCAGGGAGGCGTGGCCTGGGCGGGACTGGGGAGTGGCGAGCCCTCAGATCCTGCATATAAGCAGC TGCTTTTTGCCTGTACTGG...
Embodiment 2
[0045] Example 2 Transfection of umbilical cord mesenchymal stem cells with mir-338 overexpressing lentivirus
[0046] 1. Isolation, culture and identification of human umbilical cord mesenchymal stem cells
[0047] HUMSCs were cultured in DMEM low-glucose medium containing 10% FBS, and the cells were cultured in a 37°C, 5% carbon dioxide incubator. The cells were observed with an inverted microscope once a day, and the culture medium was changed every 2 to 4 days. When the cells in the culture flask grow to 80% to 95% confluence, perform cell passage or collect cells for plating. Discard the old culture medium, add 2 mL of 0.25% trypsin digestion solution, after the cells become round and float, add 4 mL of culture medium to terminate the digestion, then transfer to a sterile centrifuge tube, centrifuge at 1000 r / min for 4 min, discard the supernatant, Transfer to a new sterile culture bottle at a ratio of 1:4 to 1:6.
[0048] Discard the old medium in the culture flask, w...
Embodiment 3
[0059] Example 3 Construction and identification of silent mir-338 lentiviral particles
[0060] 1. Target gene acquisition
[0061] The lentivirus system vector LentiCRISPR v2 was used to connect the cDNA sequence of the target gene to the vector. The restriction enzyme cut site: BamHI / Apa I was used to obtain the target plasmid LentiCRISPR v2-mir338 by gene synthesis. The sequence of the recombinant plasmid LentiCRISPR v2-mir338 As shown in SEQ ID NO:2.
[0062] SEQ ID NO: 2
[0063]TGATGCGGTTTTGGCAGTACATCAATGGGCGTGGATAGCGGTTTGACTCACGGGGATTTCCAAGTCTCCACCCCATTGACGTCA ATGGGAGTTTGTTTTGGCACCAAAATCAACGGGACTTTCCAAAATGTCGTAACAACTCCGCCCCATTGACGCAAATGGGCGGTAGGCG TGTACGGTGGGAGGTCTATATAAGCAGCGCGTTTTGCCTGTACTGGGTCTCTCTGGTTAGACCAGATCTGAGCCTGGGAGCTCTCTGG CTAACTAGGGAACCCACTGCTTAAGCCTCAATAAAGCTTGCCTTGAGTGCTTCAAGTAGTGTGTGCCCGTCTGTTGTGTGACTCTGGT AACTAGAGATCCCTCAGACCCTTTTAGTCAGTGTGGAAAATCTCTAGCAGTGGCGCCCGAACAGGGACTTGAAAGCGAAAGGGAAACC AGAGGAGCTCTCTCGACGCAGGACTCGGCTTGCTGAAGCGCGCACGGCAAGAGGCGAGG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com