Method used for separating and/or extracting radioactive metal cations
A metal cation and radioactive technology, which is applied in the field of water treatment containing radioactive metal cations, can solve the problems of unreached residue, poor selectivity, and low adsorption amount, and achieve the effect of low residue, high removal rate, and high adsorption amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
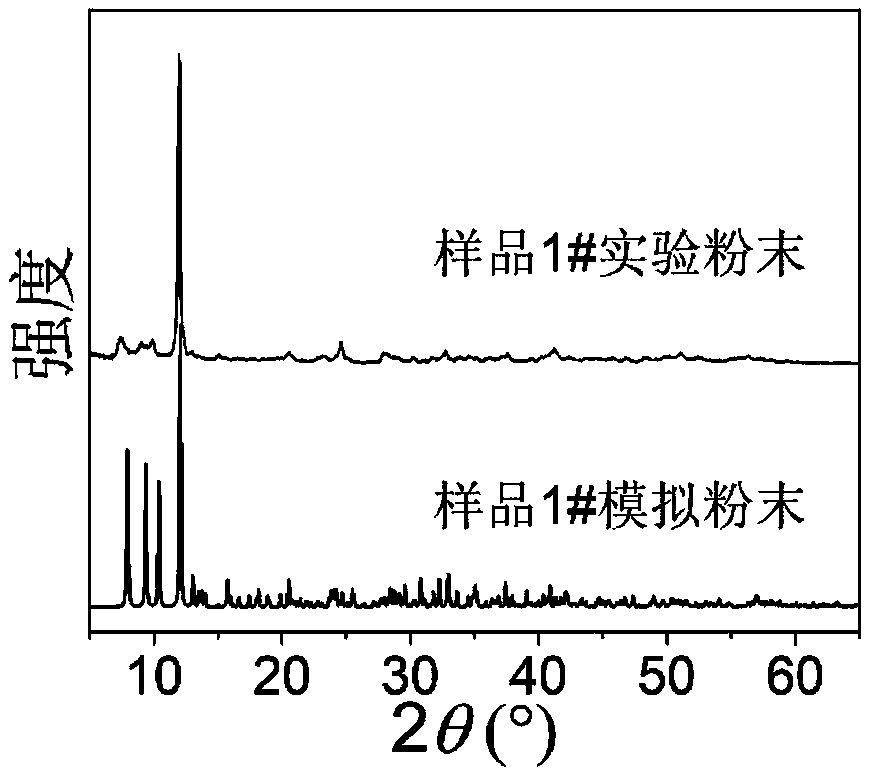
[0057] [Sn 3 S 7 ] n 2n- The preparation of the two-dimensional skeleton material refers to Example 1 in the Chinese patent application whose publication number is CN104399538, and the selected [Sn 3 S 7 ] n 2n- The two-dimensional framework material is a compound [Me 2 NH 2 ] 4 / 3 [Me 3 NH] 2 / 3 sn 3 S 7 1.25H 2 O, which is only described in this application [Sn 3 S 7 ] n 2n- A typical representative of two-dimensional framework materials, described in this application [Sn 3 S 7 ] n 2n- Two-dimensional framework materials are not limited thereto. [Me 2 NH 2 ] 4 / 3 [Me 3 NH] 2 / 3 sn 3 S 7 1.25H 2 The synthetic method of O (sample 1#) is specifically:
[0058] Mix tin source, sulfur source, dimethylamine aqueous solution and water according to a certain molar ratio, fully stir at room temperature, seal it into a stainless steel reactor lined with polytetrafluoroethylene, react at a certain temperature for a period of time, and then naturally cool down ...
Embodiment 2
[0063] Embodiment 2 utilizes [Sn 3 S 7 ] n 2n- Exchange Kinetics Test of Two-Dimensional Framework Materials for the Removal and Recovery of Radioactive Metal Cations
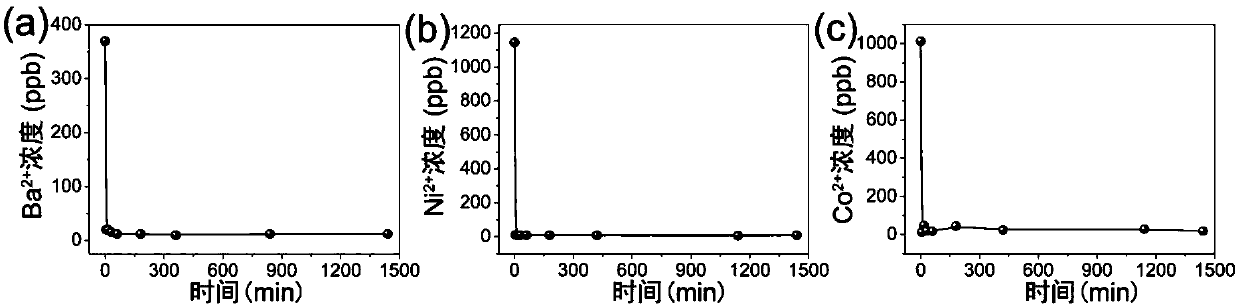
[0064] Sample 1# to Ba 2+ 、Ni 2+ 、Co 2+ The specific steps of the ion exchange kinetics experiment are as follows: the ground powder sample 1# respectively reacts with a certain initial concentration of Ba 2+ 、Ni 2+ 、Co 2+ The aqueous solution of ions is at 25°C, under the condition of V (solution volume): m (mass of exchanger) = 1000mL / g, the supernatant is taken at regular intervals to measure the ion concentration therein. The result is as figure 2 As shown, it can be seen from the figure that the sample 1# is to Ba 2+ 、Ni 2+ 、Co 2+ The ion exchange can reach equilibrium within 5 minutes, and the residual amount reaches the WHO standard for drinking water.
Embodiment 3
[0065] Embodiment 3 utilizes [Sn 3 S 7 ] n 2n- Adsorption model testing of two-dimensional framework materials for the removal and recovery of radioactive metal cations
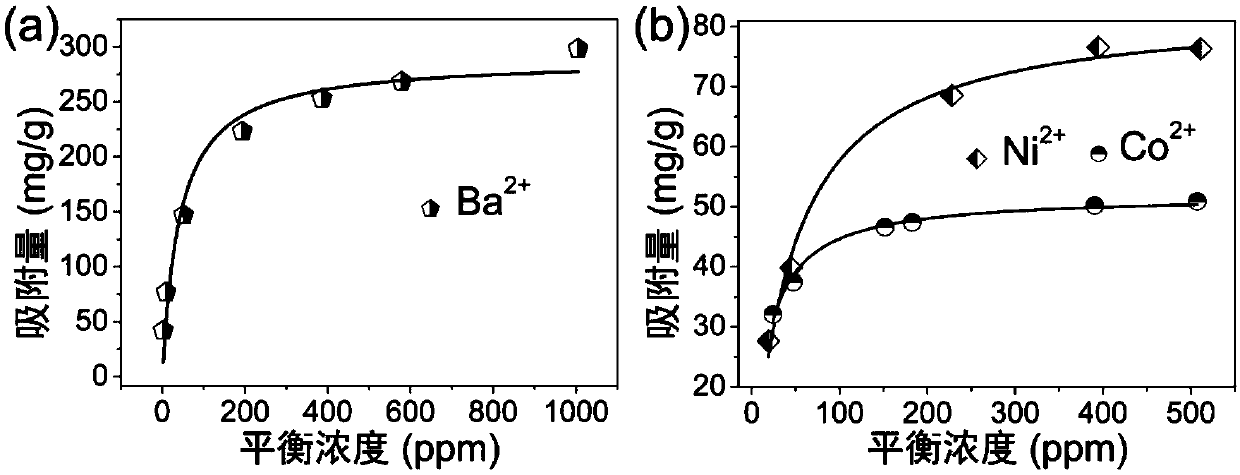
[0066] Sample 1# to Ba 2+ 、Ni 2+ 、Co 2+ The specific steps of the ion adsorption model experiment are as follows: the ground powder sample 1# is subjected to different initial concentrations of Ba 2+ 、Ni 2+ 、Co 2+ The ionic aqueous solution was exchanged for 24 hours at 25°C, V (solution volume): m (mass of exchanger) = 1000mL / g, and the supernatant and initial solution were taken to measure the ion concentration. The result is as image 3 As shown, it can be seen from the figure that the sample 1# is to Ba 2+ 、Ni 2+ 、Co 2+ The adsorption capacity of ions can reach 289.04mg / g, 83.64mg / g, 51.98mg / g respectively.
[0067] Sample 1# to Ba 2+ The adsorption amount of ions reached 148% of the theoretical value. Using X-ray photoelectron spectroscopy to exchange Ba 2+ 、Ni 2+ 、Co 2+ Sample 1# befor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com