Chemical synthesis method of Bacillus pyocyaneus 011 serotype O antigen oligosaccharide
A Pseudomonas aeruginosa, synthesis method technology, applied in the field of chemical synthesis of Pseudomonas aeruginosa O11 serotype O antigen oligosaccharides, to achieve the effect of simple and efficient method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
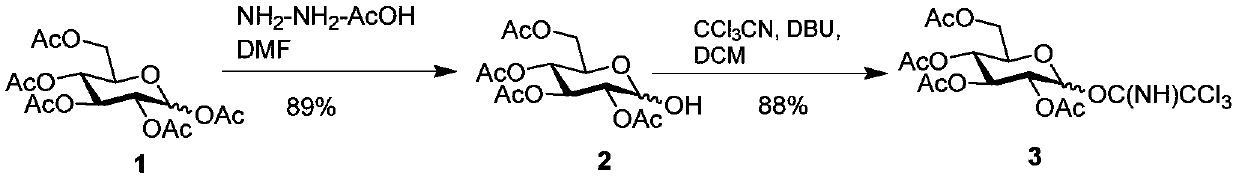
[0048] The synthesis of sugar building block 3 (D-glucose building block) is as follows figure 1 :
[0049] like figure 1 shown, starting with peracetylglucose 1, in dry N,N-dimethylformamide using hydrazine acetate (NH 2 -NH 2 -AcOH) selectively hydrolyzes the acetyl group at position 1. The resulting 1-OH glucose 2 was then purified in anhydrous dichloromethane using trichloroacetonitrile (Cl 3 CN) and 1,8-diazabicycloundec-7-ene (DBU) to give glucosyl trichloroacetimide ester 3.
[0050] Specific test operations and steps:
[0051] Compound 2: Peracetylglucose 1 (5.0 g, 12.8 mmol) was dissolved in dry DMF (65 ml), and hydrazine acetate (1.45 g, 15.6 mmol) was added. The reaction was stirred at 40°C for 6 h, and the disappearance of the reaction was monitored by TLC. The reaction solution was cooled to room temperature, washed with saturated NaCI solution, separated organic phase, washed with anhydrous NaCI 2 SO 4 It was dried, concentrated and purified by silica ge...
Embodiment 2
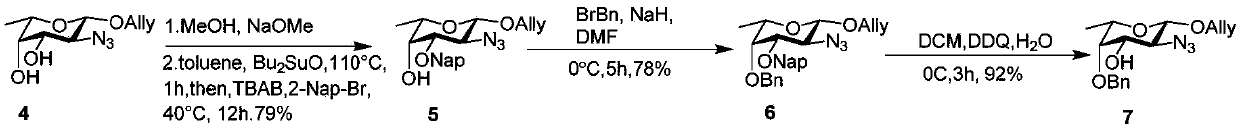
[0054] The synthetic route of sugar building block 7 (L-fucose building block) is figure 2 .
[0055] like figure 2 As shown, using allyl 2-deoxy-2 azido-L-fucoside 4 as the starting material, firstly removing the acetyl groups at positions 3 and 4 in an alkaline environment containing sodium methoxide, the obtained Under the action of dibutyltin oxide and bromomethylnaphthalene, the dihydroxy compound is selectively protected with Nap group at the 4th position to obtain compound 5, and the hydroxyl group of 3-Nap compound 5 is protected with benzyl group to generate compound 6. Finally, the Nap group of 6 was removed with DDQ to obtain compound 3-OH compound 7.
[0056] Specific test operations and steps:
[0057] Compound 5: Allyl 2-deoxy-2azido-L-fucoside 4 (500 mg, 2.2 mmol) Anhydrous toluene (22 ml) followed by addition of dibutyltin oxide (814 mg, 3.3 mmol). The reaction solution was heated to 110 °C and stirred for 1 h. Subsequently, the reaction solution was coo...
Embodiment 3
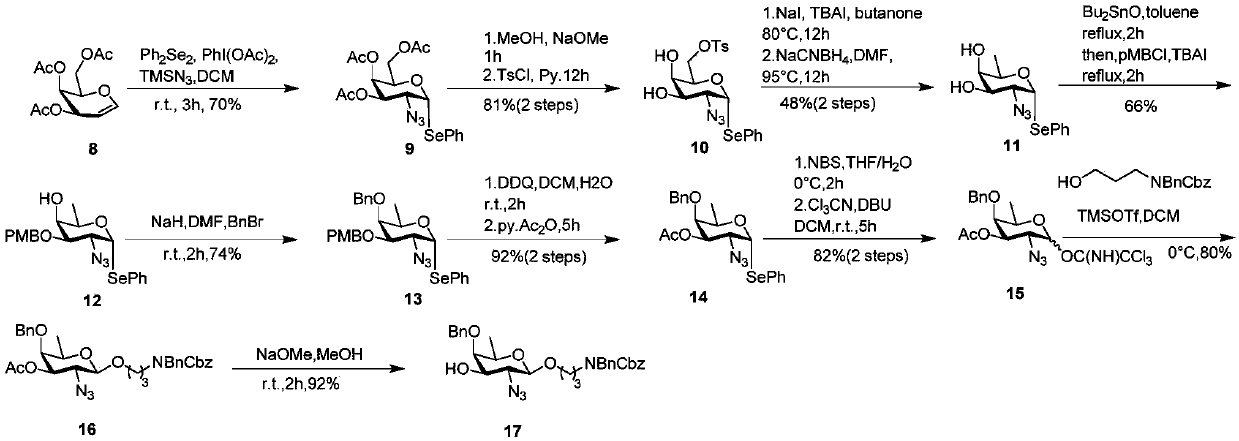
[0062] The synthetic route of sugar block 17 is image 3 .
[0063] like image 3 As shown, starting from 3,4,6-O-triacetylmannosene 8, and iodobenzene diacetate (PhI(OAc) 2 ), azidotrimethylsilane (TMS-N 3 ) and diphenyldiselenide (Ph 2 Se 2 ) addition reaction to obtain 1-selenophenyl-2-azido compound 9. The three acetyl groups of 9 were removed with methanol and sodium methoxide, and then the hydroxyl group at position 6 was selectively protected with 4-toluenesulfonyl chloride (TsCl) to obtain compound 10. Next, the Ts group of 6-Ts glucose 10 was iodized with sodium iodide, followed by sodium cyanoborohydride (NaCNBH 3 ) to reduce the 6th position to a methyl group to obtain D-fucose compound 11. With p-methoxybenzyl chloride (PMBCl) and dibutyltin oxide (Bu 2 SnO) selectively protects the 3-position of the D-fucose compound to obtain compound 12, and then protects the 4-position hydroxyl with a benzyl group to obtain compound 13. The 4-position Nap of 13 is remov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com