Method for separating and purifying human serum albumin from Cohn component V supernatant
A technology for separation and purification of human serum albumin, applied in the preparation methods of serum albumin, albumin peptides, peptides, etc., can solve the problems of long operation time and large amount of buffer, and achieves low cost, reduced dosage, and improved The effect of plasma utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This embodiment separates and purifies human serum albumin by the following method
[0050] Equilibrate the anion-exchange chromatography medium DEAE Sepharose Fast Flow with 20mM phosphate buffer with a pH value of 7.0, adjust the pH value of the Cohn component V supernatant to 7.0, and load it into the chromatography column. Rinse 3 CVs with phosphate buffered saline;
[0051] Use a pH value of 4.0 and a concentration of 20 mM citric acid-sodium citrate buffer to elute the chromatography column, collect the eluate, and concentrate the obtained elution peak by ultrafiltration with an ultrafiltration membrane with a molecular weight cut-off of 10 kDa to obtain human serum Albumin; the chromatography medium was finally washed with 20 mM citric acid-sodium citrate buffer, pH 4.0, containing 0.5 M NaCl.
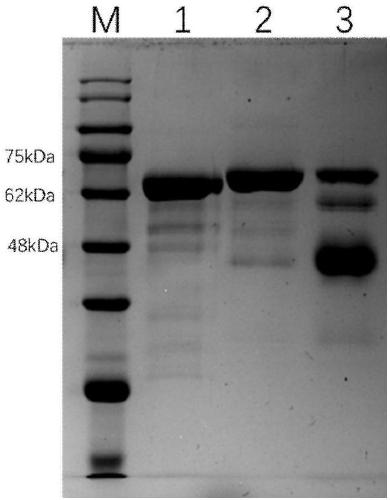
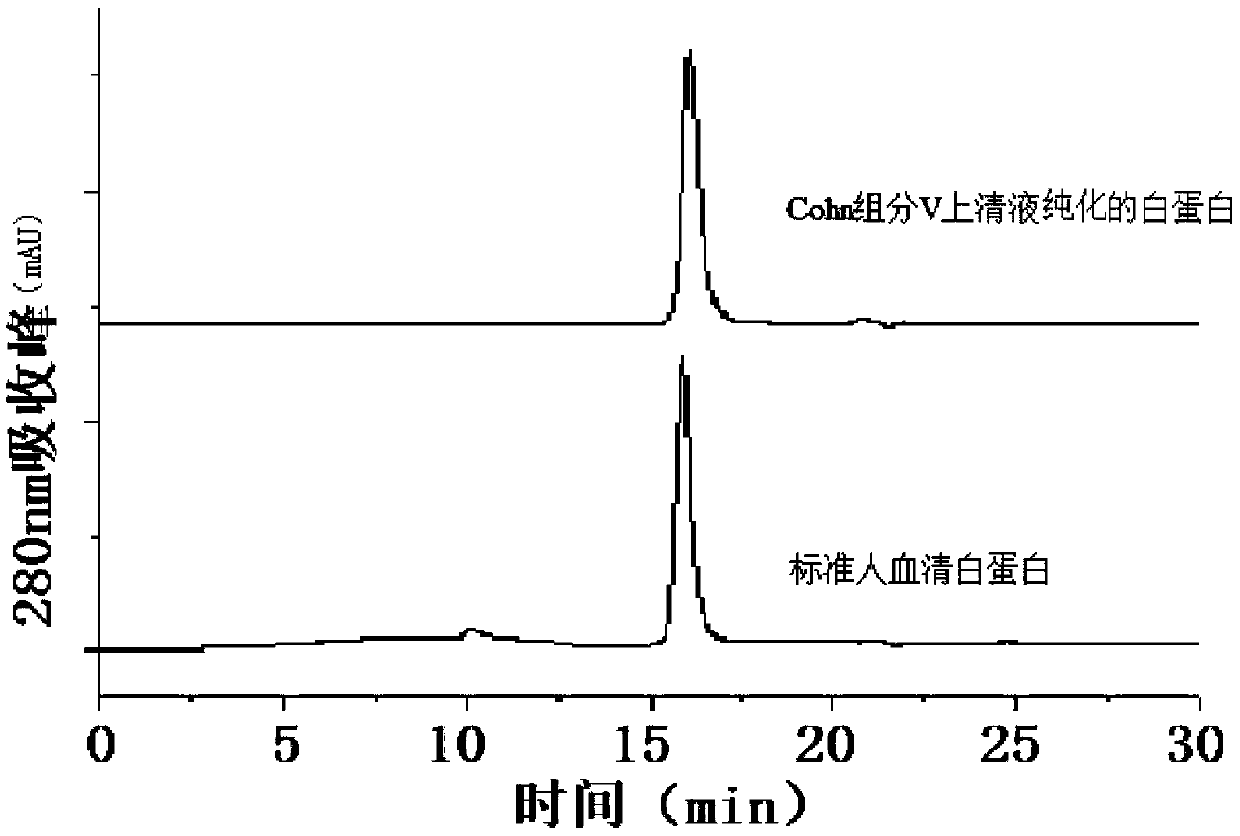
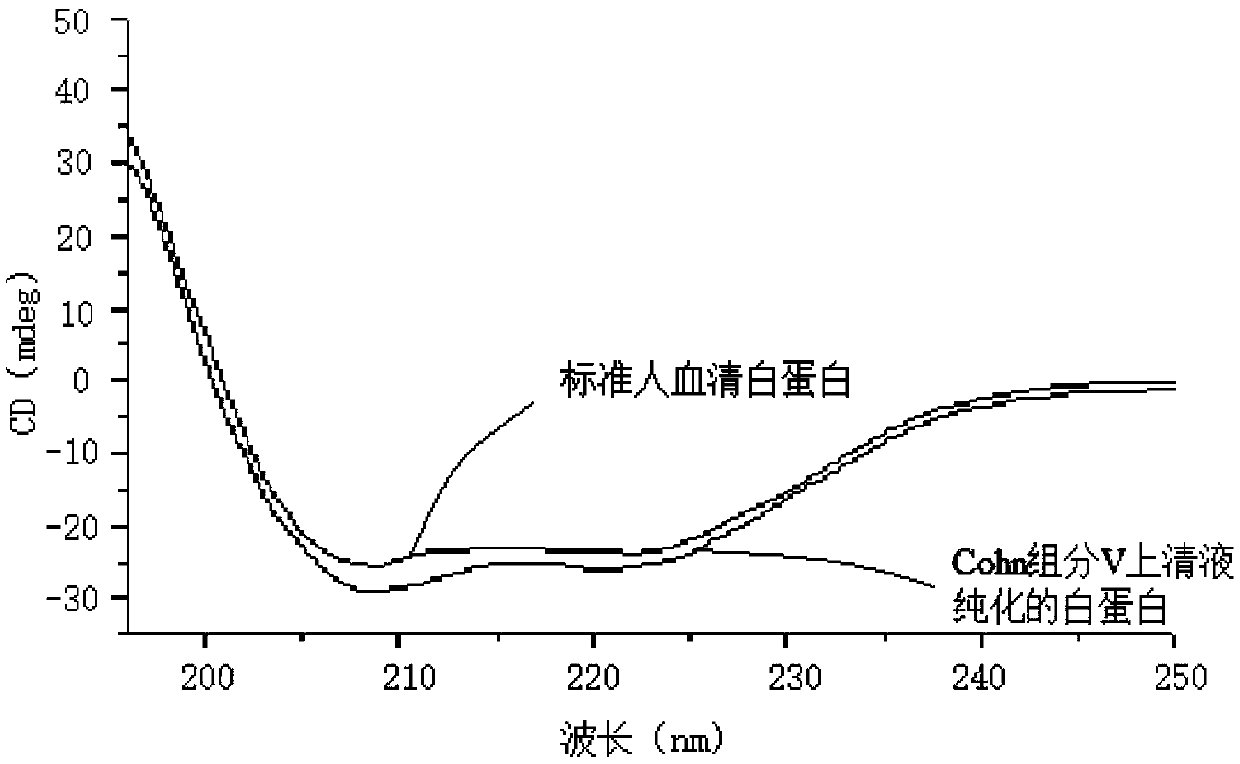
[0052] The human serum albumin that embodiment 1 obtains is carried out 12%SDS-PAGE detects, and specific result is as follows figure 1 As shown, the result shows a sin...
Embodiment 2
[0055] This embodiment separates and purifies human serum albumin by the following method
[0056] Equilibrate the anion-exchange chromatography medium Q Sepharose Fast Flow with 20mM phosphate buffer with a pH value of 7.5, adjust the pH value of the Cohn component V supernatant to 7.5, and load it into the chromatography column. Rinse 5 CVs with phosphate buffered saline;
[0057] Use a pH value of 4.5 and a concentration of 50 mM citric acid-sodium citrate buffer to elute the chromatography column, collect the eluate, and concentrate the obtained elution peak by ultrafiltration with an ultrafiltration membrane with a molecular weight cut-off of 15 kDa to obtain human serum Albumin; the chromatography medium was finally washed with 50 mM citric acid-sodium citrate buffer, pH 4.5, containing 1 M sodium chloride.
Embodiment 3
[0059] This embodiment separates and purifies human serum albumin by the following method
[0060] Equilibrate the anion-exchange chromatography medium Capto DEAE with 20mM phosphate buffer with a pH value of 6.0, adjust the pH value of the Cohn component V supernatant to 6.0, and load it into the chromatography column. Wash with salt buffer for 3 CVs;
[0061] Use a pH value of 4.0 and a concentration of 20 mM citric acid-sodium citrate buffer to elute the chromatography column, collect the eluate, and concentrate the obtained elution peak by ultrafiltration with an ultrafiltration membrane with a molecular weight cut-off of 10 kDa to obtain human serum Albumin; the chromatography medium was finally washed with 30 mM citric acid-sodium citrate buffer, pH 4.0, containing 0.2 M NaCl.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com