Method for enzymatic synthesis of penicillin V salt
A technology of penicillin V and enzymatic synthesis, which is applied in the field of medicine and chemical industry to achieve the effects of good product quality, low cost and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
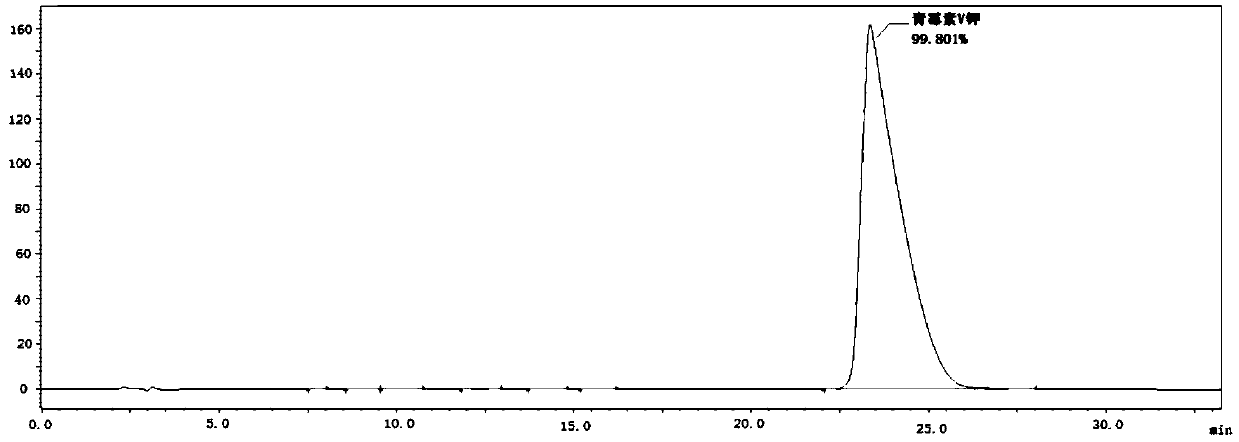
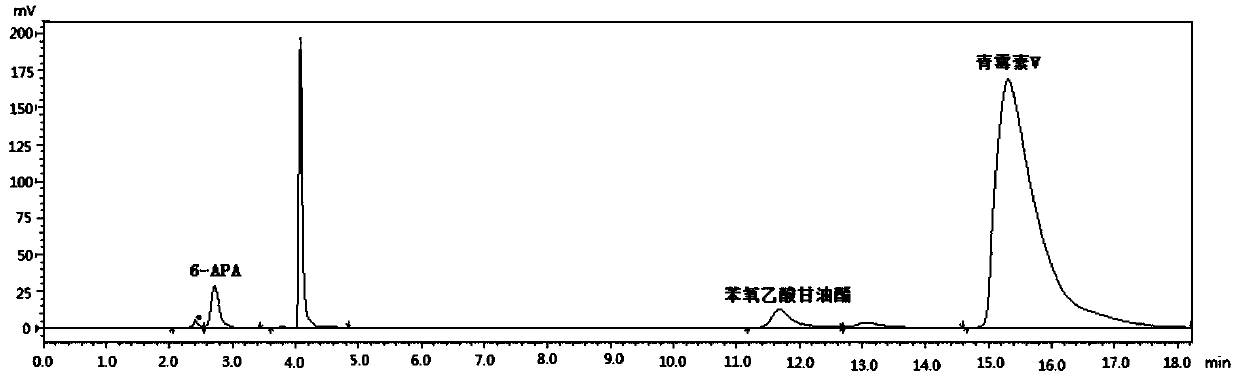
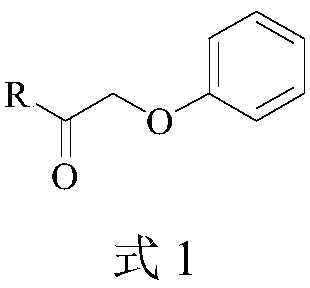
[0032]Take 25.0g 6-APA, add 300mL pure water, drop 3mol / L ammonia solution to fully dissolve 6-APA, add 30.0g ethylene glycol phenoxyacetate, mix well, add 50.0g immobilized penicillin acylase , add dropwise 3mol / L ammonia solution to make the reaction pH 5.0, temperature 30°C, react for 90 minutes and take samples for HPLC detection. At this time, the concentration of 6-APA is 0.8mg / mL, and the concentration of penicillin V is 106.9mg / mL; Separate the immobilized enzyme and the reaction solution, adjust the pH value of the reaction solution to 3.0 with 6mol / L hydrochloric acid, grow the crystal for 20 minutes, and filter to obtain the penicillin V acid filter cake; the filter cake is transferred to 5 times the weight of pure water, and the Potassium carbonate solution of a certain concentration adjusts the pH value of the mixed solution to 6.0, then adds n-butanol to a certain concentration, evaporates and concentrates under reduced pressure until crystals are separated out, a...
Embodiment 2
[0034] Take 25.0g 6-APA, add 400mL pure water, add dropwise 3mol / L ammonia solution to fully dissolve 6-APA, add 30.0g phenoxyacetate glyceride, mix well, add 75.0g immobilized penicillin acylase, drop Add 3 mol / L ammonia solution to make the reaction pH 6.0, temperature 20°C, react for 80 minutes and take samples for HPLC detection. At this time, the concentration of 6-APA is 0.3 mg / mL, and the concentration of penicillin V is 87.5 mg / mL; catalyze and reaction solution, adjust the pH value of the reaction solution to 2.8 with 6mol / L hydrochloric acid, grow crystals for 20 minutes, and filter to obtain penicillin V acid filter cake; transfer the filter cake to 4 times the weight of pure water, potassium carbonate solution to adjust the pH value of the mixed solution to 6.3, then add n-butanol to a certain concentration, evaporate and concentrate under reduced pressure until crystals are precipitated, and the crystals are filtered, washed, and dried to obtain 39.1 g of penicilli...
Embodiment 3
[0036] Take 25.0g 6-APA, add 400mL pure water, add dropwise 3mol / L ammonia solution to fully dissolve 6-APA, add 35.0g phenoxyacetate butanetetrayl ester, mix well, add 50.0g immobilized penicillin acylase , add dropwise 3mol / L ammonia solution to make the reaction pH 5.0, temperature 30°C, react for 90 minutes and sample HPLC for detection. At this time, the concentration of 6-APA is 0.5mg / mL, and the concentration of penicillin V is 89.0mg / mL; Separate the immobilized enzyme and the reaction solution, adjust the pH value of the reaction solution to 2.8 with 6mol / L hydrochloric acid, grow the crystal for 20 minutes, filter to obtain the penicillin V acid filter cake; transfer the filter cake to 4 times the weight of pure water, and use Potassium carbonate solution of a certain concentration adjusts the pH value of the mixed solution to 6.5, then adds n-butanol to a certain concentration, evaporates and concentrates under reduced pressure until crystals are precipitated, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com