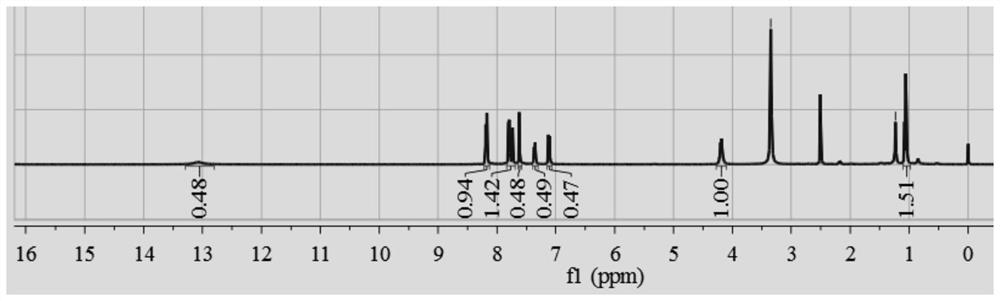
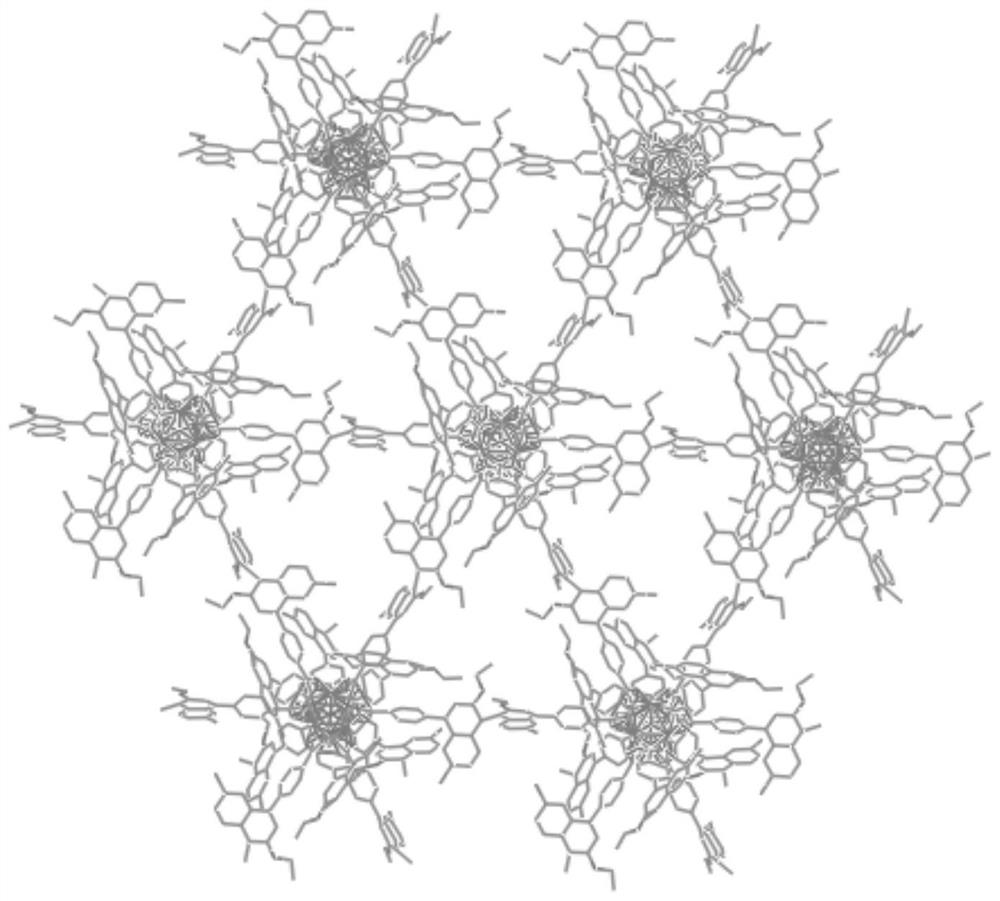
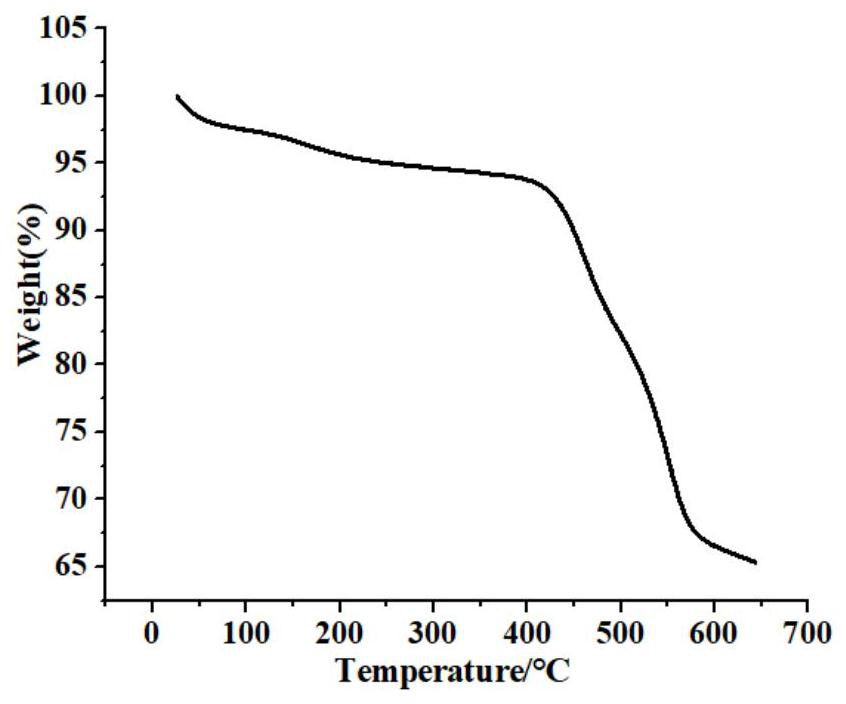
A kind of preparation method and application of chiral zr-mof catalyst
A catalyst and chirality technology, applied in the field of chiral catalyst preparation, can solve the problems of poor chiral selectivity and cannot be reused, and achieve the effects of short reaction time, easy recovery and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 (R)-6, the synthesis of 6'-dibromo-1,1'-bi-2-naphthol
[0058] Weigh the raw material R-1,1'-bi-2-naphthol (10.0mmol, 2.86g) into a 100ml three-necked flask, add 20.0g of dichloromethane, cool to 0°C, and slowly add liquid bromine ( 25.2mmol, 4.0g), after the dropwise addition was completed, the reaction solution was light orange. Maintain the temperature and continue the reaction for 24h, then add Na 2 S 2 o 3 (7.4mmol, 1.20g) in aqueous solution, continue to stir for 2h. After the reaction was completed, the color of the reaction solution changed from orange to light yellow. Filter the reaction solution, transfer the filtrate to a separatory funnel, wash the organic phase three times with a saturated NaCl solution, combine the organic phases, and add an appropriate amount of NaCl 2 SO 4 After drying, the solvent was removed by rotary evaporation to obtain (R)-6,6'-dibromo-1,1'-bi-2-naphthol as a light yellow solid product.
Embodiment 2
[0059] Example 2 (R)-6,6'-dibromo-2,2'-diethoxy-1,1'-binaphthyl synthesis
[0060] Weigh the raw material (R)-6,6'-dibromo-1,1'-bi-2-naphthol (10.0mmol, 4.44g) into a 100ml flask, add 40.0ml of acetone, stir until the solids are completely dissolved, Then add anhydrous K 2 CO 3 (40.0mmol, 5.50g), bromoethane (60.0mmol, 7.00g), the reaction system was heated to reflux, and the temperature was maintained for 48h. After the reaction was completed and dropped to room temperature, filtered, and the filtrate was rotary evaporated under reduced pressure to obtain the yellow solid product (R)-6,6'-dibromo-2,2'-diethoxy-1,1'-binaphthyl .
Embodiment 3
[0061] Embodiment 3 (R)-6, the synthesis of 6'-dichloro-2,2'-diethoxy-1,1'-binaphthalene
[0062] N 2 Under protection, the raw material (R)-6,6'-dibromo-2,2'-diethoxy-1,1'-binaphthalene (10.0mmol, 4.97g), cuprous chloride (22.0mmol, 2.20g), DMF 15.0ml was placed in a 100ml three-neck flask, and stirred at a constant temperature of 110°C for 48h. After the reaction was completed, it was filtered while it was hot, and the filtrate was poured into 300ml of secondary water while stirring, and a pale yellow solid was precipitated. After filtration, the solid was dried and dissolved in dichloromethane. After column chromatography (eluent: dichloromethane), the product 6,6'-dichloro-2,2'-diethoxy-1,1 was obtained as a yellow solid '-binaphthyl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com