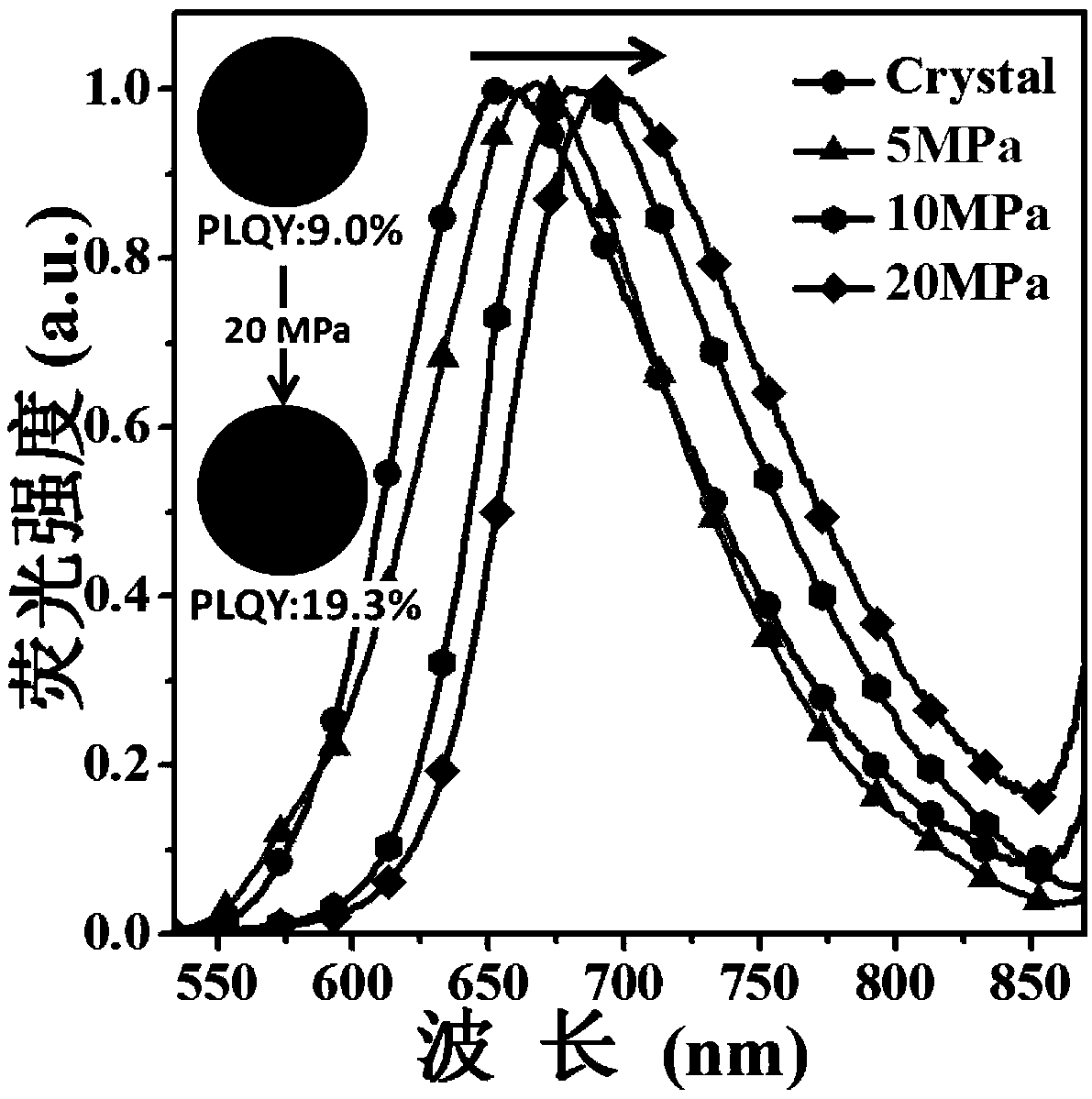
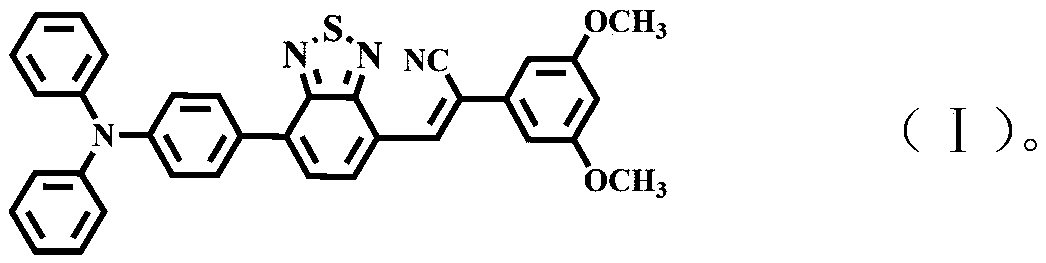
Diazosulfide benzyl cyanide derivative and preparation method and application thereof
A technology of benzothiadiazole phenylacetonitrile and its derivatives, which is applied in the field of benzothiadiazole phenylacetonitrile derivatives and their preparation, achieving the effect of high contrast and obvious color change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention discloses a preparation method of a force-induced fluorescence enhancement material, which comprises the following steps:
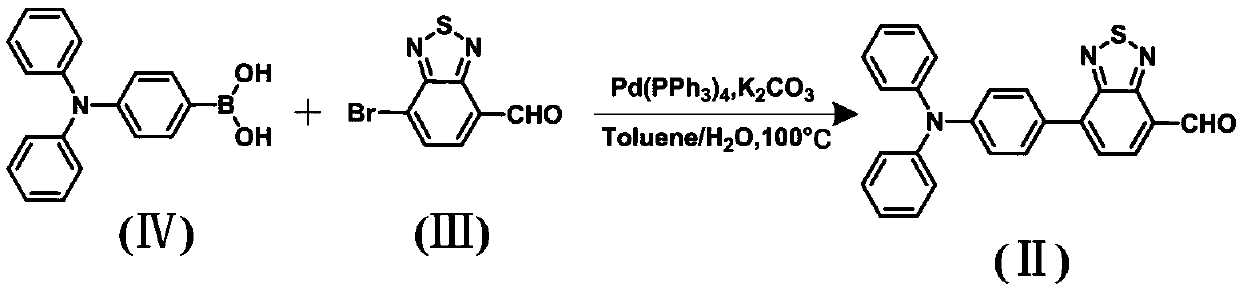
[0026] Step 1, the synthesis of intermediate (Ⅱ):
[0027] Its synthetic route is as follows:
[0028]
[0029] Weigh 7-bromobenzene-1,2,5-thiadiazole-4-carbaldehyde (Ⅲ), triphenylamine boric acid (Ⅳ), tetrakistriphenylphosphopalladium and potassium carbonate solution and dissolve them in chromatographic toluene and tetrahydrofuran, wherein , The molar ratio of 7-bromobenzene-1,2,5-thiadiazole-4-carbaldehyde (Ⅲ) to triphenylamine boric acid (Ⅳ) is 1:1-1:1.5; 7-bromobenzene-1,2, 5-thiadiazole-4-carboxaldehyde: potassium carbonate: chromatographic toluene: chromatographic tetrahydrofuran is 10mmol: 0.8-1.2mmol: 50-60ml: 30-40ml; under the protection of nitrogen atmosphere, the reaction time is 12-24h under reflux and stirring. The temperature is 80-100°C, and when a large amount of red solid particles are precipitated, the reaction...
Embodiment 1
[0037] Synthesis of intermediate (Ⅱ): Weigh 1.3g (6mmol) of 7-bromobenzene-1,2,5-thiadiazole-4-carbaldehyde (Ⅲ), 1.8g (6.2mmol) of triphenylamine boric acid (Ⅳ), 0.25g (0.22mmol) tetraphenylphosphopalladium and potassium carbonate solution (3M) were dissolved in chromatographic toluene (50ml) and tetrahydrofuran (30ml). When the solid particles are separated out, click on the plate to determine the reaction progress and terminate the reaction. After the reaction, all the crude products were dissolved with dichloromethane, transferred, washed with water, and the organic phase was taken, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation, and the powdered product and the coarse silica gel powder were mixed and loaded into the column 1.8 g of the product intermediate (II) was obtained with a yield of 75%.
[0038] The characterization data are as follows: 1 H NMR (400MHz, CDCl 3 )δ10.62(s,1H),9.32(d,J=7.2Hz,1H),9.04-9.02(m,3H),7.39(t,J=8H...
Embodiment 2
[0042] Synthesis of intermediate (Ⅱ): Weigh 2.11g (10mmol) of 7-bromobenzene-1,2,5-thiadiazole-4-carbaldehyde (Ⅲ), 2.92g (10.1mmol) of triphenylamine boric acid (Ⅳ) ), tetrakistriphenylphosphopalladium 0.25g (0.22mmol) and potassium carbonate solution (3M) were dissolved in chromatographic toluene (50ml) and tetrahydrofuran (30ml), under nitrogen atmosphere protection, reflux stirring reaction time is 20h, wait for When a large amount of red solid particles precipitated, spot the plate to determine the reaction progress and terminate the reaction. After the reaction, all the crude products were dissolved with dichloromethane, transferred, washed with water, and the organic phase was taken, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation, and the powdered product and the coarse silica gel powder were mixed and loaded into the column , using dichloromethane and petroleum ether at a ratio of 1:2 as the eluent for column chromatography to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com