Preparation method of blood coagulation factor X activator RVV-X and prepared RVV-X
A technology of coagulation factor, RVV-X, applied in the direction of biochemical equipment and methods, enzymes, peptidases, etc., can solve the conventional research that limits the scope of RVV-X market use, RVV-X is difficult to mass-produce, and the filler is physically stable problems such as poor performance, to achieve the effect of maintaining the volume and structural stability of the column bed, which is conducive to scale-up production and has fewer preparation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of blood coagulation factor X activator RVV-X comprises the following steps:
[0027] 1) Viper venom is dissolved in buffer, separated by molecular sieve chromatography column, eluted with buffer, monitored with a nucleic acid protein detector, and the elution line is drawn according to the A280nm value, and the chromogenic substrate method is used to detect RVV- The elution peak of Ⅹ activity is collected and concentrated;
[0028] 2) The active peak concentrate in step 1) is separated by a weak cationic column, and gradient eluted with a buffer solution containing 0-0.5mol / L NaCl, monitored by a nucleic acid protein detector, and the elution line is drawn according to the A280nm value, The elution peaks with RVV-X activity were collected respectively by chromogenic substrate detection.
[0029] Step 1) of the preparation method of the present invention uses viper venom as a raw material, dissolves it in a buffer, conducts rough separation throu...
Embodiment 1
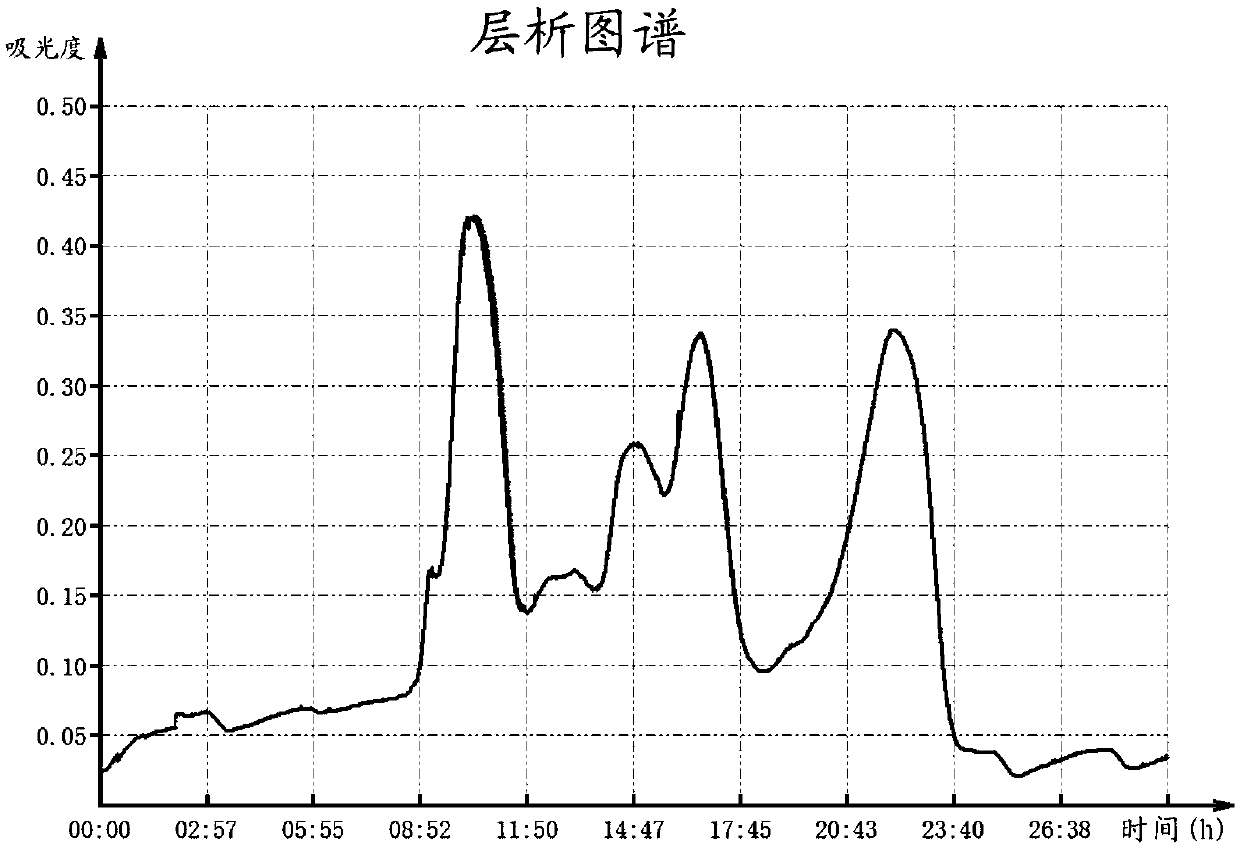
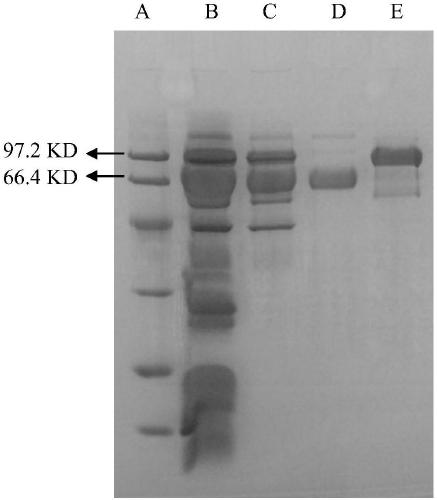
[0056] Dissolve 100 mg of Viper venom in 1.5 mL of 0.05 mol / L TRIS-HCl buffer (pH 7.4), centrifuge at 12,000 rpm for 10 min, take the supernatant and load it onto a Sephacryl S-100HR column that has been equilibrated with the above buffer, and continue to add buffer Liquid elution, flow rate 0.2ml / min, automatic collection of eluent. Monitor with a nucleic acid protein detector, draw the elution line according to the A280nm value (see figure 1 ), while using the chromogenic substrate method to detect and determine the elution peak with RVV-X activity. The results showed that three peaks were mainly obtained in this step of separation, and the peaks with RVV-X activity were collected and concentrated for electropherogram analysis, which showed that most of the small molecular substances were removed in this step.
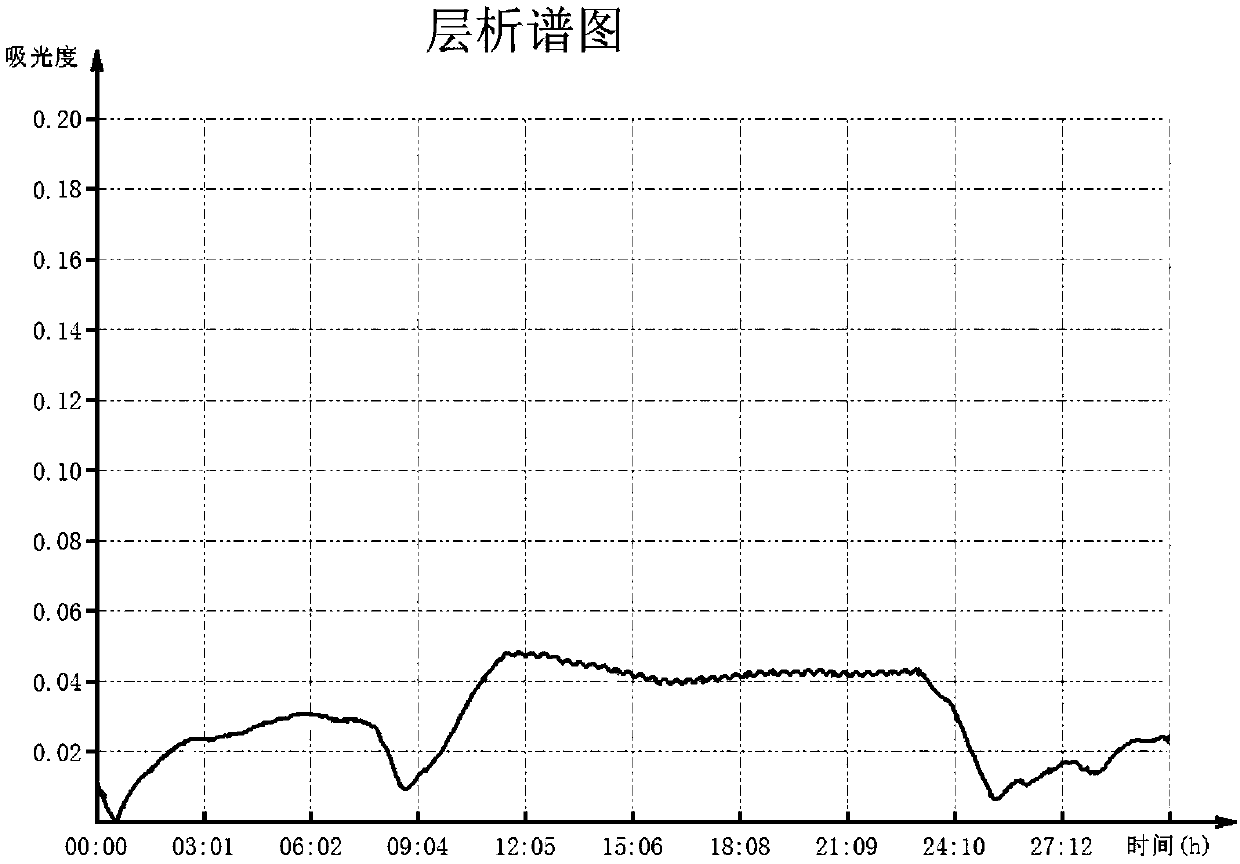
[0057] The peak with RVV-X activity was concentrated and loaded onto a weak cationic CM Sepharose Fast Flow column equilibrated with 0.05mol / L ammonium acetate buff...
Embodiment 2
[0059] Dissolve 100mg of Viper venom in 1.5mL 0.05mol / L TRIS-HCl buffer solution (pH 7.4), centrifuge at 12000rpm for 10min, take the supernatant and load it onto a Superdex 200 column equilibrated with the above buffer solution, and continue to add buffer solution for elution , the flow rate is 0.2mL / min, and the eluate is collected automatically. Monitor with a nucleic acid protein detector, draw the elution line according to the A280nm value, and use the chromogenic substrate method to detect and determine the elution peak with RVV-X activity. result with figure 1 Similarly, three peaks were mainly obtained in this step of separation, and the peaks with RVV-X activity were collected and concentrated for electropherogram analysis. It can be seen that most of the small molecular substances were removed in this step.
[0060] The peak with RVV-X activity was concentrated and loaded onto a weak cationic CM Sepharose Fast Flow column equilibrated with 0.05mol / L ammonium acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com