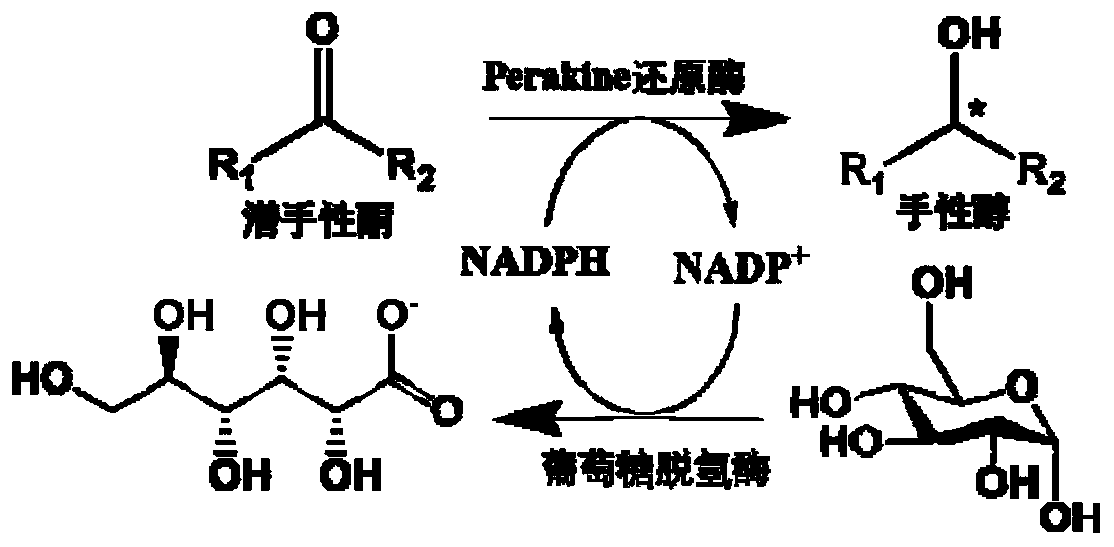
A kind of method applying perakine reductase to synthesize chiral alcohol
A technology of reductase and chiral alcohol, which is applied in fermentation and other fields, can solve the problems that chiral alcohol cannot be widely used, and achieve the effects of easy preparation, good stability and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The expression of embodiment 1Perakine reductase
[0041] The Perakine reductase expression vector was constructed in previous research (Sun L, Ruppert M, Sheludko Y, Warzecha H, Zhao Y, J. Purification, cloning, functional expression and characterization of perakine reductase: the first example from the AKR enzyme family, extending the alkaline network of the plantRauvolfia. Plant Mol Biol. 2008, 67(5): 455-67.), heat shock conversion to E.coli M15 competent cells were used to obtain engineering bacteria expressing Perakine reductase. The engineering bacteria expressing Perakine reductase were inoculated into the LB medium containing 50 μg / ml ampicillin and 25 μg / ml kanamycin and cultured with shaking at 37°C for 12 hours. Transfer to 1L LB medium containing the same concentration of ampicillin and kanamycin, when the optical density of the culture medium is OD 600 When it reaches 0.6, add isopropyl-β-D-thiogalactopyranoside (IPTG) with a final concentration of 0.3m...
Embodiment 2
[0042] The expression of embodiment 2 glucose dehydrogenase
[0043] The glucose dehydrogenase gene (GenBank: D10626.1) encoding Bacillus megaterium IAM1030 is shown in the sequence listing GDH-DNA, and the amino acid sequence is shown in the sequence listing GDH-AA. The glucose dehydrogenase gene was fully synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and then connected to the pET-28a(+) vector. After sequencing and verifying the sequence, it was transformed into E.coli BL21(DE3) competent cells by heat shock to obtain glucose Dehydrogenase expression engineering bacteria. The glucose dehydrogenase-expressing engineered bacteria were inoculated into LB medium containing 50 μg / ml kanamycin and cultured with shaking at 37°C for 12 hours. Transfer to 1L LB medium containing the same concentration of kanamycin, when the optical density of the culture medium OD 600 When it reaches 0.6, add IPTG with a final concentration of 0.1mM to induce at 20°C for 16h, centrifuge...
Embodiment 3
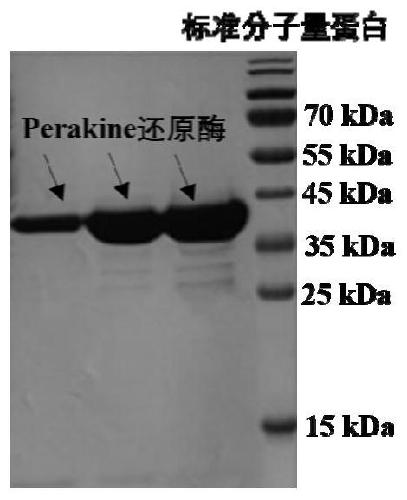
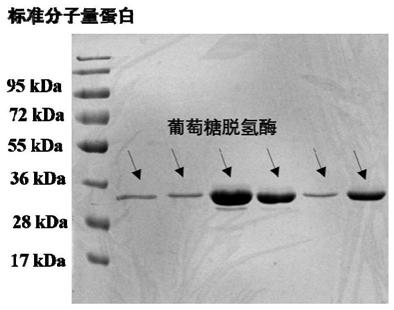
[0044] Embodiment 3Perakine reductase and the purification of glucose dehydrogenase
[0045] Perakine reductase-expressing engineered bacteria or glucose dehydrogenase-expressed engineered bacteria cells were resuspended in 20ml lysate (10mM imidazole, 50mM NaH 2 PO 4 , 300mM NaCl, pH 8.0), shake well and add 1-2mg / ml lysozyme, after ice bath for 40min, put ultrasonic breaker 3 times, 3min each time, each interval of 15min. The broken liquid was centrifuged at 22000×g for 50 min, and the obtained supernatant was the crude enzyme liquid. Use Ni-NTA as the purification material, the column volume is 3ml, 15ml lysate equilibrates the Ni-NTA column, loads the sample crude enzyme solution at a rate of 1ml / min, rinses with buffer (20mM imidazole, 50mM NaH 2 PO 4 , 300mM NaCl, pH8.0) to remove unadsorbed protein, and finally eluted with elution buffer (250mM imidazole, 50mM NaH 2 PO 4 , 300mM NaCl, pH8.0) to collect the target protein by elution, and use 5L Kpi buffer (50mM KH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com