1,2-oxygen azetidines compound and preparation method and application thereof
A technology of heterocyclobutanes and compounds, which is applied in the field of 1,2-oxazetidine compounds and their preparation, can solve the problems of poor functional group tolerance, poor chemoselectivity, poor atom economy, etc., and achieve high yield , good regioselectivity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
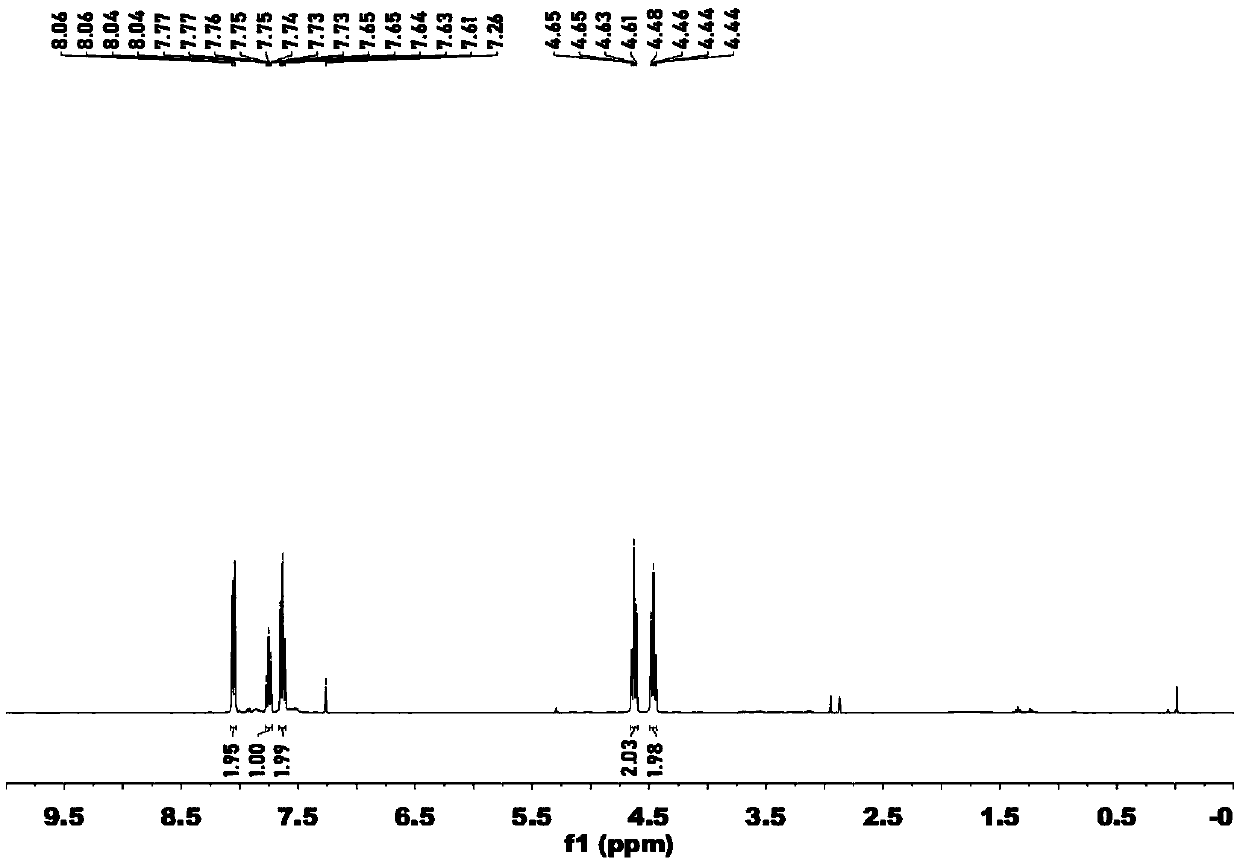
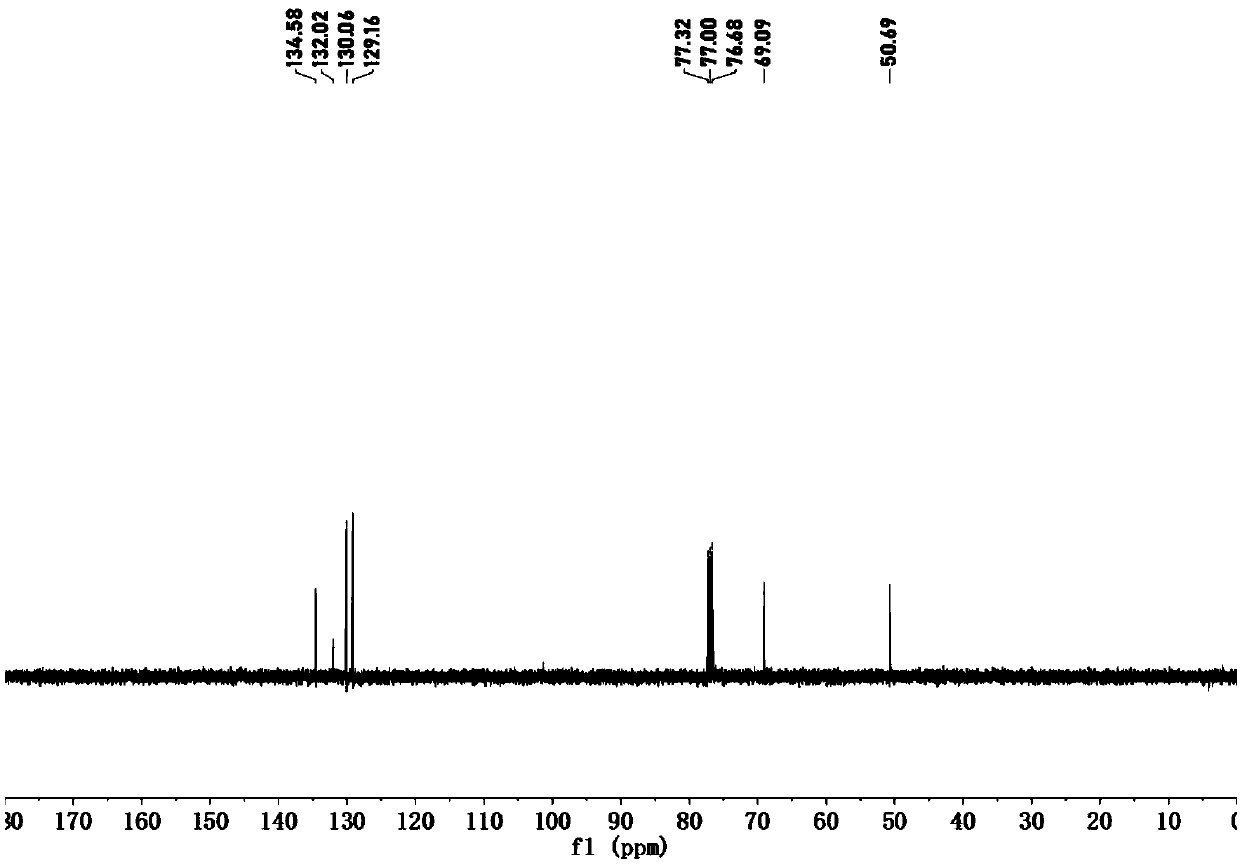
[0055] Example 1 Preparation of 1,2-oxazetidine compounds
[0056] (1) Add 120mmol 1,2-dibromoethane and 120mmol triethylamine to 80mL N,N-dimethylformamide solution containing 60mmol N-hydroxyphthalimide, and stir at room temperature until the reaction solution Become colorless, collect the precipitated solid in the reaction solution, dilute the filtrate with ice water and filter out the precipitated solid, combine the precipitated solid and recrystallize the precipitate with ethanol, which is compound III;
[0057] (2) Add 15 mL of hydrobromic acid with a mass concentration of 48% to 120 mmol of compound III in 10 mL of glacial acetic acid, stir and react at 130° C. for 5 minutes, cool, filter, and concentrate the filtrate to obtain yellow compound IV;
[0058] (3) Add 5 mL of pyridine to 8.7 mmol of compound IV, stir and react at room temperature for 5 minutes, then add 20.9 mmol of 4-methylbenzenesulfonyl chloride to the reaction solution, stir at room temperature for 5 ho...
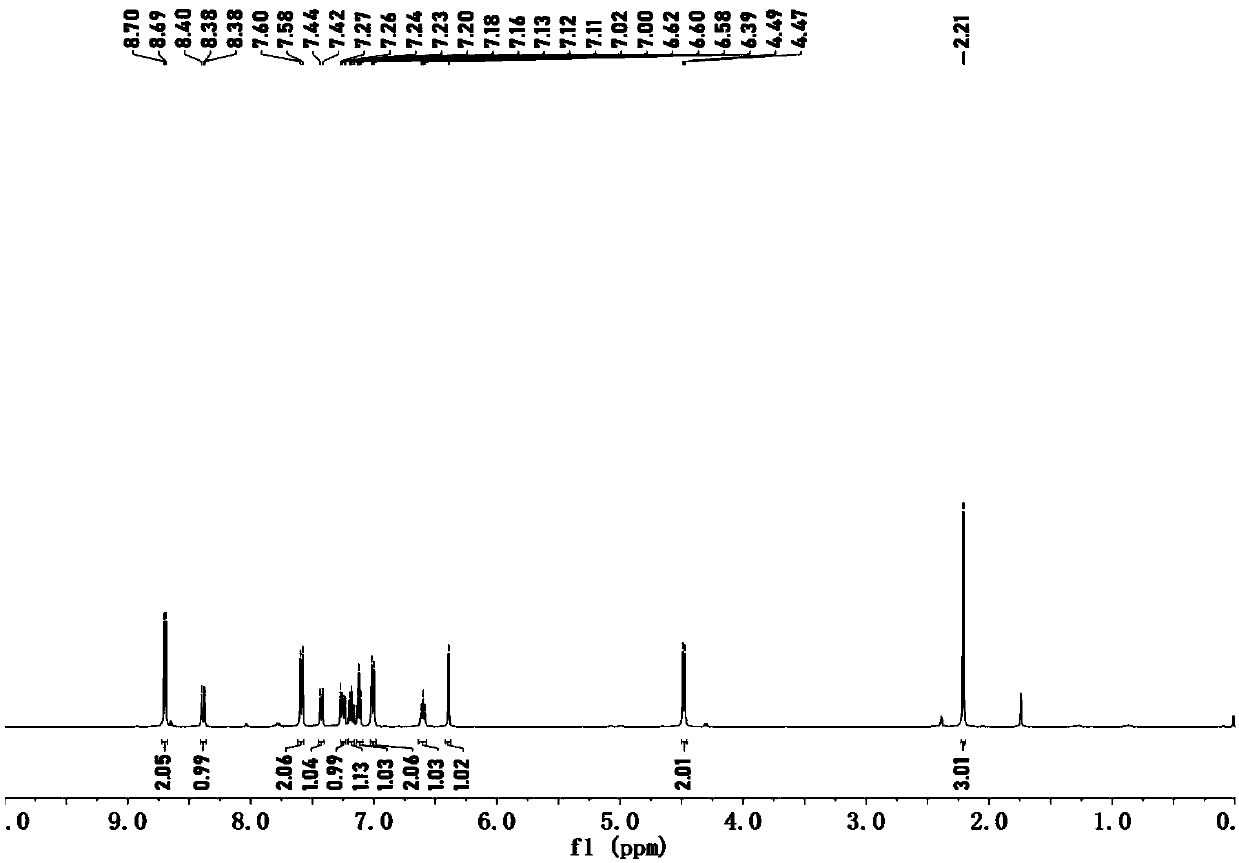
Embodiment 2
[0062] (1) Add 120mmol 1,2-dibromoethane and 120mmol triethylamine to 80mL N,N-dimethylformamide solution containing 60mmol N-hydroxyphthalimide, and stir at room temperature until the reaction The liquid becomes colorless, collect the precipitated solid in the reaction solution, dilute the filtrate with ice water and filter out the precipitated solid, combine the precipitated solid and recrystallize the precipitate with ethanol, which is compound III;
[0063] (2) Add 15 mL of hydrobromic acid with a mass concentration of 48% to 120 mmol of compound III in 10 mL of glacial acetic acid, stir and react at 130° C. for 5 minutes, cool, filter, and concentrate the filtrate to obtain yellow compound IV;
[0064] (3) Add 5 mL of pyridine to 8.7 mmol of compound IV, stir and react at room temperature for 5 minutes, add 20.9 mmol of benzenesulfonyl chloride to the reaction solution, stir at room temperature for 5 hours, then add 1.0 M hydrochloric acid dropwise to the reaction solution...
Embodiment 3
[0067] (1) Add 120mmol 1,2-dibromoethane and 120mmol triethylamine to 80mL N,N-dimethylformamide solution containing 60mmol N-hydroxyphthalimide, and stir at room temperature until the reaction The liquid becomes colorless, collect the precipitated solid in the reaction solution, dilute the filtrate with ice water and filter out the precipitated solid, combine the precipitated solid and recrystallize the precipitate with ethanol, which is compound III;
[0068] (2) Add 15 mL of hydrobromic acid with a mass concentration of 48% to 120 mmol of compound III in 10 mL of glacial acetic acid, stir and react at 130° C. for 5 minutes, cool, filter, and concentrate the filtrate to obtain yellow compound IV;
[0069] (3) Add 5 mL of pyridine to 8.7 mmol of compound IV, stir and react at room temperature for 5 minutes, add 20.9 mmol of p-fluorobenzenesulfonyl to the reaction solution, stir at room temperature for 5 hours, then add 1.0 M hydrochloric acid solution to neutrality, extracted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com