Method for synthesizing 7,21-dyhydroxyl-20-methyl pregnane-4-alkene-3-ketone by microorganisms
A technology for microbial synthesis and methylpregnant, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of low substrate conversion rate, long conversion cycle, single hydroxylation product, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
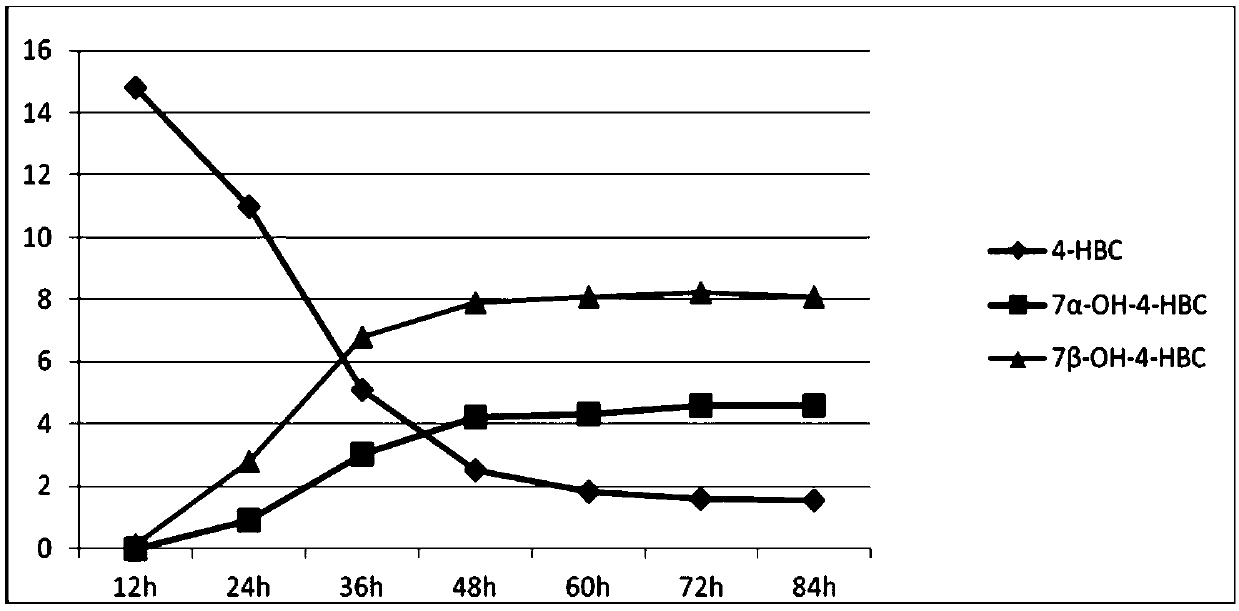
[0039] Example 1 Method for synthesizing 7α- and 7β-hydroxyl-4-HBC by Mucor circinelloides lusitanicus bacteria GB1
[0040] 1. Preparation of cell liquid culture of Mucor circiniferus strain GB1
[0041] Pick a ring of Mucor circiniferii strain GB1 on the solid medium, inoculate it in a Erlenmeyer flask with seed medium, and culture it on a shaker at 200r / min at 28°C until the logarithmic growth phase. That is, the cell liquid culture of Mucor circinifolia was obtained.
[0042] 2. Shake flask fermentation of Mucor circinelloides lusitanicus strain
[0043] The above-mentioned Mucor circinelloides lusitanicus (Mucor circinelloides lusitanicus) cell culture solution is inserted into the fermentation medium with an inoculum size of 8% (v / v), and the filling volume is 30ml fermentation medium in a 250ml Erlenmeyer flask, and the culture temperature is 25- At 30°C, the bacteria were cultured at a rotational speed of 220r / min to enter the logarithmic growth phase.
[0044] 3. B...
Embodiment 2
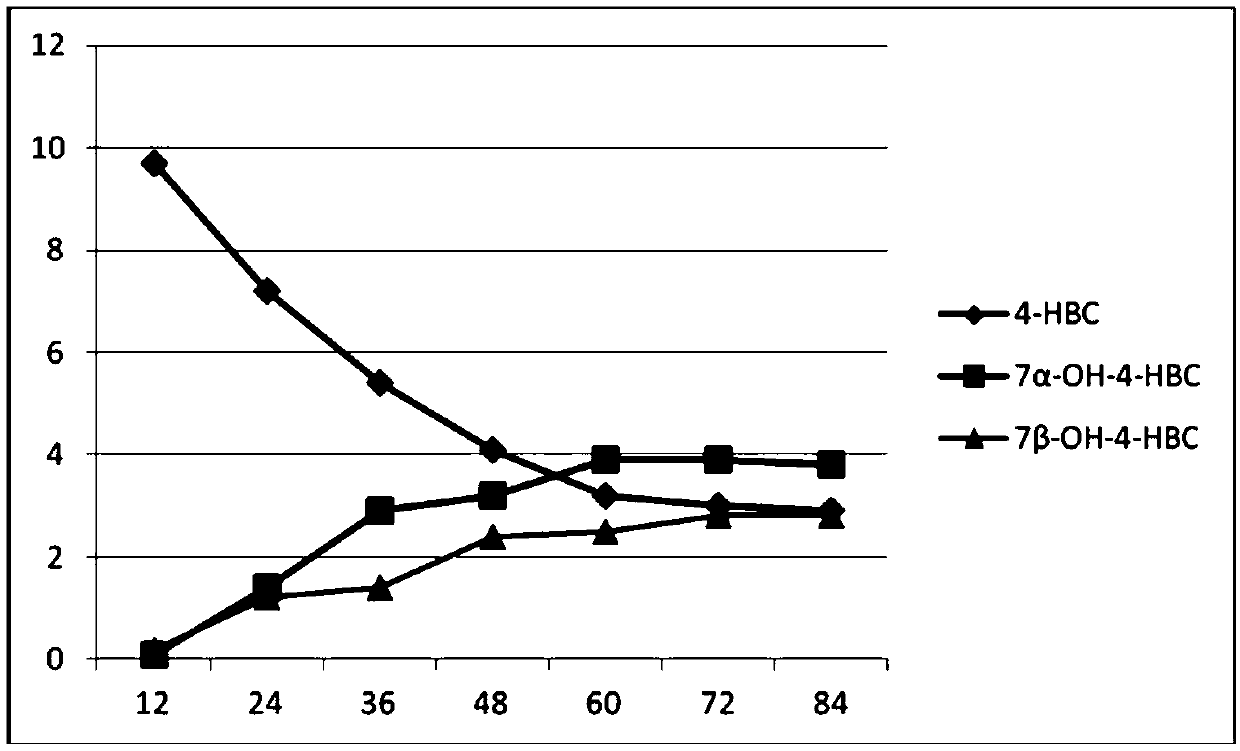
[0056] Embodiment 2 Acremonium strictum (Acremonium strictum) GB2 synthesizes the method for 7α- and 7β-hydroxyl-4-HBC
[0057] 1. Preparation of cell liquid culture of Acremonium erectus strain GB2
[0058] Pick a ring of Acremonium erecta strain GB2 on the solid medium, inoculate it in a Erlenmeyer flask with seed medium, and culture it on a shaker at 200r / min at 28°C until logarithmic phase. That is, the cell liquid culture of Acremonium erectum was obtained.
[0059] 2. Shake flask fermentation of Acremonium strictum (Acremonium strictum) bacterial strain
[0060] The above-mentioned upright Acremonium strictum (Acremonium strictum) cell culture solution is inserted into the fermentation medium with an inoculum size of 8% (v / v), and the filling volume is 30ml of the fermentation medium in a 250ml Erlenmeyer flask, and the culture temperature is 25- The bacteria were cultured at 30°C at a rotational speed of 200r / min to enter the logarithmic growth phase. 3. Biotransform...
Embodiment 3
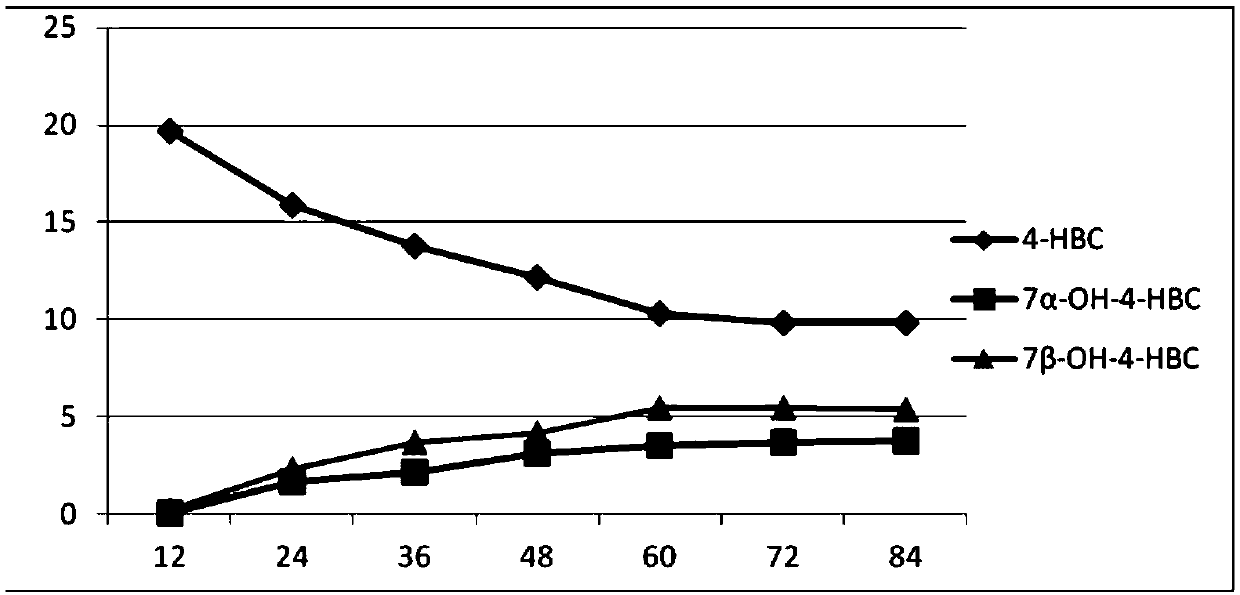
[0072] Example 3 Method for Synthesizing 7α- and 7β-Hydroxyl-4-HBC from Mucor.racemosus GB3
[0073] 1. Prepare the cell liquid culture of Mucor.racemosus GB3
[0074] Pick a ring of Mucor racemosus strain GB3 on the solid medium, inoculate it in a Erlenmeyer flask with seed medium, and culture it on a shaker at 200r / min at 28°C until the logarithmic phase, namely A liquid culture of cells of Mucor racemosum was prepared.
[0075] 2. Shake flask fermentation of Mucor.racemosus strain
[0076] The above-mentioned Mucor.racemosus (Mucor.racemosus) cell culture solution was inserted into the fermentation medium with an inoculum size of 8% (v / v). At 30°C, the bacteria were cultured at a rotational speed of 200r / min to enter the logarithmic growth phase.
[0077] 3. Biotransformation of 4-HBC by Mucor.racemosus strain
[0078] Accurately weigh an appropriate amount of substrate 4-HBC, put it into the cell fermentation broth in step 2, make the final concentration 20g / l, transfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com