Lyophobic matrix sustained-release tablet for pH independence drugs and preparation method thereof
A technology of dependent, sustained-release tablets, applied in the directions of drug delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve the problems of incomplete pH-dependent drug release, etc., and achieve easy industrial scale-up and actual production, promotion Dissolving and improving the effect of in vitro release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation method of diclofenac sodium sustained-release tablets (100 mg specification)
[0038]Tablet weight 291.3mg, diclofenac sodium 100mg, cetyl alcohol (Cetyl alcohol) 60.56mg, sucrose 121.09mg, povidone (PVPK30) 4.69mg, magnesium stearate 2.48mg, silicon dioxide 2.48mg. (1) Weigh the prescribed amount of cetyl alcohol in a water bath at 70°C to melt it completely, and set aside; (2) Weigh the prescribed amount of diclofenac sodium, sucrose, and povidone (PVPK30), mix well, and set aside; (3) Pour the uniformly mixed raw and auxiliary materials in (2) into the completely melted cetyl alcohol, hot-melt, mix and stir to granulate, pass through a 18-mesh sieve, let the granules cool at room temperature, add the prescribed amount of silicon dioxide and magnesium stearate, press piece.
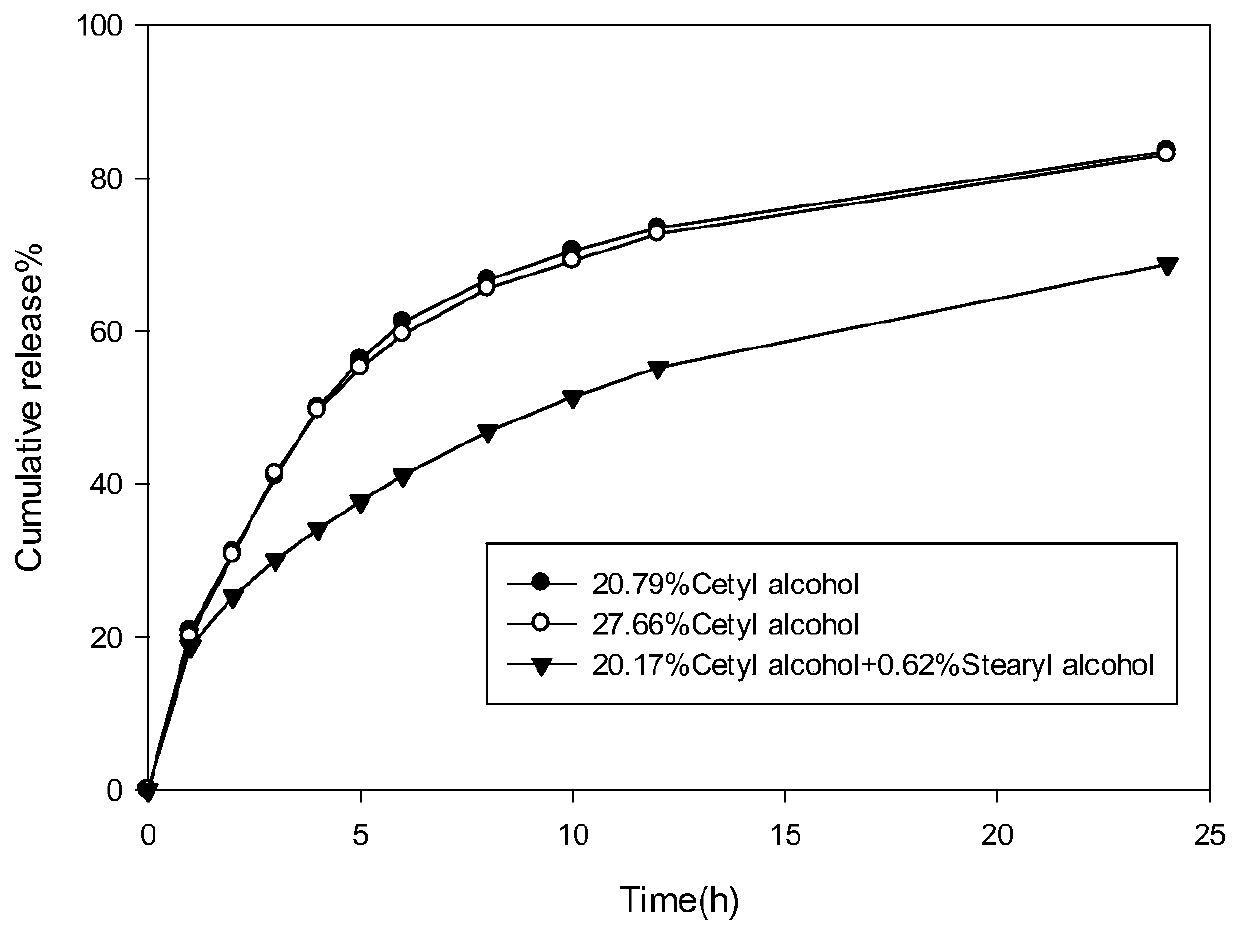
[0039] Such as figure 1 As shown, the in vitro release performance test results show that the preparation of this embodiment presents a slow drug release within 24 hours.
Embodiment 2
[0041] Preparation method of diclofenac sodium sustained-release tablets (100 mg specification)
[0042] Tablet weight 291.3mg, diclofenac sodium 100mg, cetyl alcohol 80.57mg, sucrose 101.08mg, povidone (PVPK30) 4.69mg, magnesium stearate 2.48mg, silicon dioxide 2.48mg. (1) Weigh the prescribed amount of cetyl alcohol in a water bath at 70°C to melt it completely, and set aside; (2) Weigh the prescribed amount of diclofenac sodium, sucrose, and povidone (PVPK30), mix well, and set aside; (3) Pour the uniformly mixed raw and auxiliary materials in (2) into the completely melted cetyl alcohol, hot-melt, mix and stir to granulate, pass through a 18-mesh sieve, let the granules cool at room temperature, add the prescribed amount of silicon dioxide and magnesium stearate, press piece.
[0043] Such as figure 1 As shown, the in vitro release performance test results show that the preparation of this embodiment presents a slow drug release within 24 hours.
Embodiment 3
[0045] Preparation method of diclofenac sodium sustained-release tablets (100 mg specification)
[0046] Tablet weight 291.3mg, diclofenac sodium 100mg, cetyl alcohol 58.76mg, stearyl alcohol (Stearyl alcohol) 1.81mg, sucrose 121.10mg, povidone (PVPK30) 4.69mg, magnesium stearate 2.48mg, silicon dioxide 2.48mg . (1) Weigh the prescribed amount of cetyl alcohol and stearyl alcohol in a water bath and heat them at 70°C to melt them completely, and set aside; (2) Weigh the prescribed amount of diclofenac sodium, sucrose, and povidone (PVPK30), mix them evenly, and set aside (3) Pour the uniformly mixed raw and auxiliary materials in (2) into completely melted cetyl alcohol and stearyl alcohol, heat-melt, mix and stir to granulate, pass through a 18-mesh sieve, let the granules cool at room temperature, add the prescribed amount of carbon dioxide Silicon and magnesium stearate, compressed tablet.
[0047] Such as figure 1 As shown, the in vitro release performance test results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com