Fluorine-substituted cobalt phthalocyanine/activated carbon Li/SOCl2 battery catalytic material and preparation method thereof
A catalytic material, activated carbon technology, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as inability to meet market demands, and achieve short preparation cycles. , The effect of low preparation cost and excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Take 0.619g of 4-fluorophthalic anhydride and 0.08g of which has a surface area of 1200m 2 / g of pitch coke activated carbon was mixed in a glass mortar;
[0025] 2) Take 0.6g of urea, 0.08g of ammonium molybdate and 0.4g of cobalt chloride hexahydrate and add them to the above-mentioned glass mortar and grind them thoroughly, then put them into a crucible and place them in a muffle furnace at 10°C min -1 The heating rate was raised from room temperature to 80°C and then kept for 0.2h; then at 5°C min -1 The heating rate is increased to 200 ° C, and the temperature is kept for 1 hour for sintering;
[0026] 3) Naturally cool to room temperature, wash the obtained product with ultrapure water for 10 hours, change water and wash 8 times, then use water and ethanol to filter, wash several times, and dry to obtain fluorine-substituted cobalt phthalocyanine / activated carbon Li / SOCl2 battery catalytic material.
Embodiment 2
[0028] 1) Take 0.619g of 4-fluorophthalic anhydride and 0.08g of which has a surface area of 1200m 2 / g of pitch coke activated carbon was mixed in a glass mortar;
[0029] 2) Take 0.6g of urea, 0.08g of ammonium molybdate and 0.4g of cobalt chloride hexahydrate and add them to the above-mentioned glass mortar and grind them thoroughly, then put them into a crucible and place them in a muffle furnace at 10°C min -1 The heating rate was raised from room temperature to 80°C and then kept for 0.2h; then at 5°C min -1 The heating rate is raised to 200 ° C, and the temperature is kept for 3 hours for sintering;
[0030] 3) Naturally cool to room temperature, wash the obtained product with ultrapure water for 10 hours, change water and wash 8 times, then use water and ethanol to filter, wash several times, and dry to obtain fluorine-substituted cobalt phthalocyanine / activated carbon Li / SOCl2 battery catalytic material.
Embodiment 3
[0032] 1) Take 0.819g of 4-fluorophthalic anhydride and 0.1g with a surface area of 1200m 2 / g of pitch coke activated carbon was mixed in a glass mortar;
[0033] 2) Take 1.2g of urea, 0.12g of ammonium molybdate and 0.606g of cobalt chloride hexahydrate and add them into the above-mentioned glass mortar and grind them thoroughly, then put them into a crucible and place them in a muffle furnace at 10°C min -1 The heating rate is raised from room temperature to 150°C and then kept for 0.5h; then at 10°C min -1 The heating rate is increased to 270 ° C, and the temperature is kept for 2 hours for sintering;
[0034] 3) Naturally cool to room temperature, wash the obtained product with ultrapure water for 24 hours, change the water and wash 10 times, then use water and ethanol to filter, wash several times, and dry to obtain fluorine-substituted cobalt phthalocyanine / activated carbon Li / SOCl2 battery catalytic material.
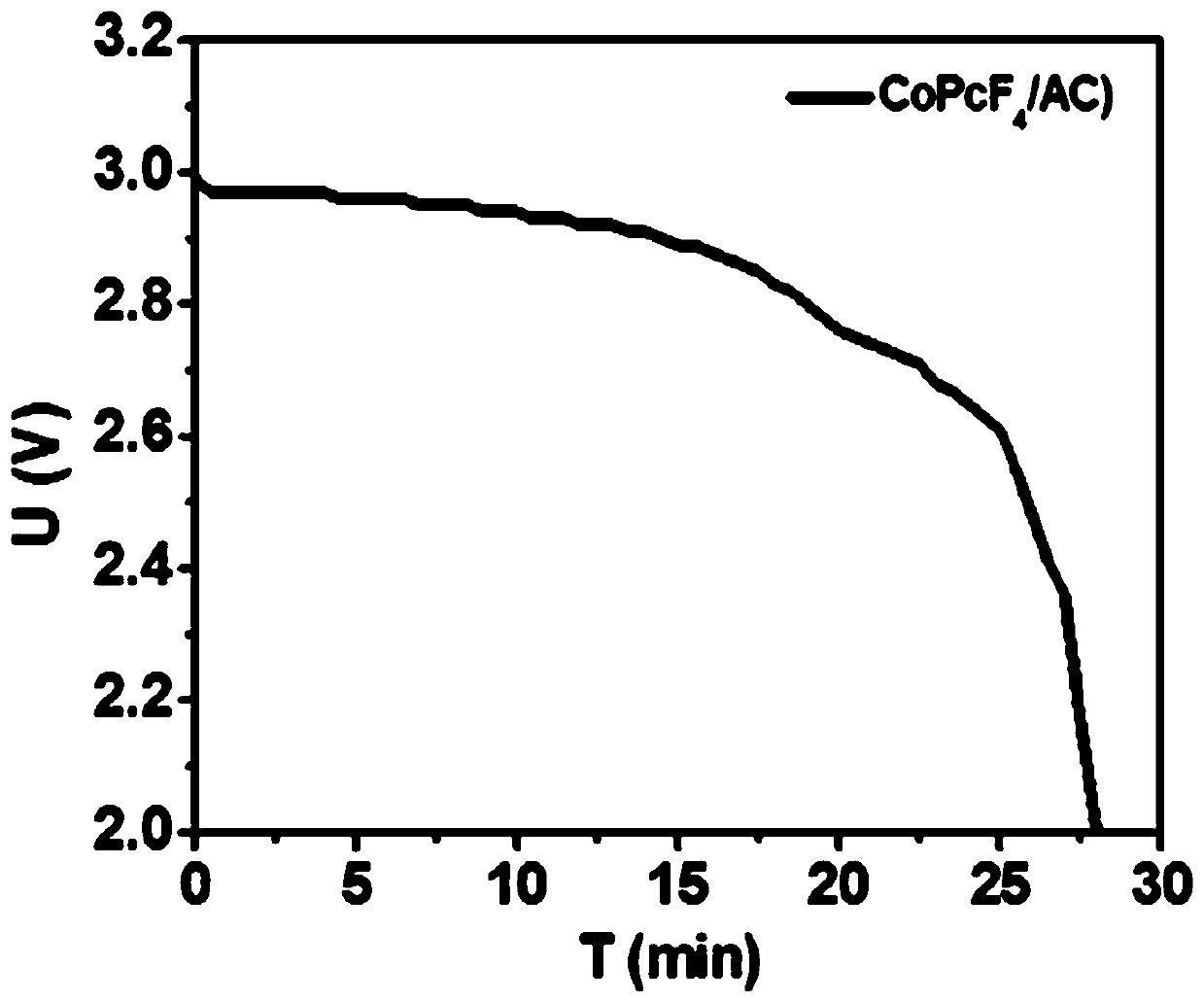
[0035] Depend on figure 1 It can be seen that at 75...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com