Condensed ring aromatic hydrocarbon based amide embedded type liquid chromatography stationary phase synthesis method
An aromatic hydrocarbon amide and liquid chromatography technology, which is applied in the field of a new approach for the synthesis of a condensed ring aromatic hydrocarbon amide embedded type liquid chromatography stationary phase, can solve the problems of complicated process, cumbersome process, many synthesis steps, etc., and achieves reduction of synthesis time and raw materials. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
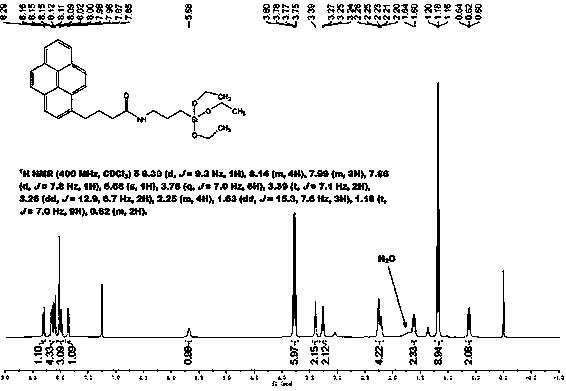
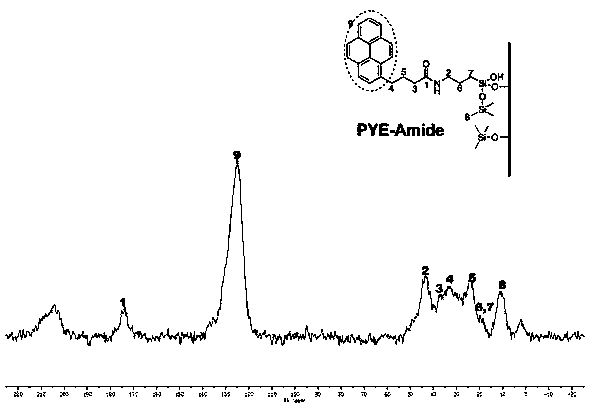
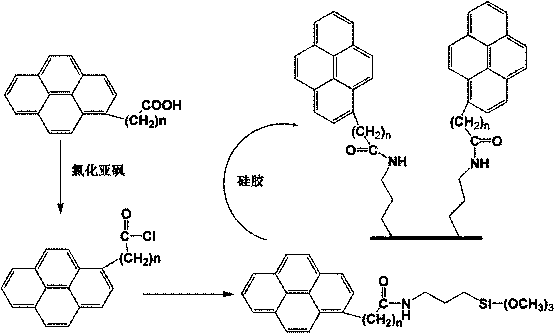
[0029] (1) Homogeneous synthesis of amide-embedded silanes
[0030] At room temperature, add pyrenylbutyric acid (8 mmol) and 3-isocyanatopropyltriethoxysilane (10 mmol) into a flask containing 60 mL of tetrahydrofuran, and then add the catalyst 4-dimethylaminopyridine in one go , continue to stir for 5 hours and then heat to reflux for 30 minutes. The solvent was removed by rotary evaporation, and then recrystallized from a benzene / n-hexane mixed solvent to obtain a tan solid, which was the synthesized pyrenylamide-embedded silane.
[0031] (2) Surface bonding modification
[0032] Disperse 5 g of vacuum-dried spherical silica at 140°C in toluene as a solvent, then add 8 mmol of pyrenylamide intercalation silane reagent synthesized, mechanically stir and reflux for 24 hours, cool and filter, and then wash the obtained solid with toluene, Wash with tetrahydrofuran, chloroform, ethanol, ethanol / water and methanol, and finally dry under vacuum at 120°C.
Embodiment 2
[0033] Embodiment 2: Change the 3-isocyanate group propyl triethoxysilane in embodiment 1 into 3-isocyanate group butyl triethoxysilane or other carbon chain length isocyanate silane, can prepare corresponding under the same conditions Pyrenylamide embedded stationary phase for liquid chromatography.
Embodiment 3
[0034] Example 3: The pyrenyl butyric acid in Example 1 was changed to anthracenyl butyric acid, the stationary phase matrix spherical silica was changed to spherical titanium oxide, and the rest of the conditions were the same to prepare an anthracenyl amide embedded liquid chromatography stationary phase .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com