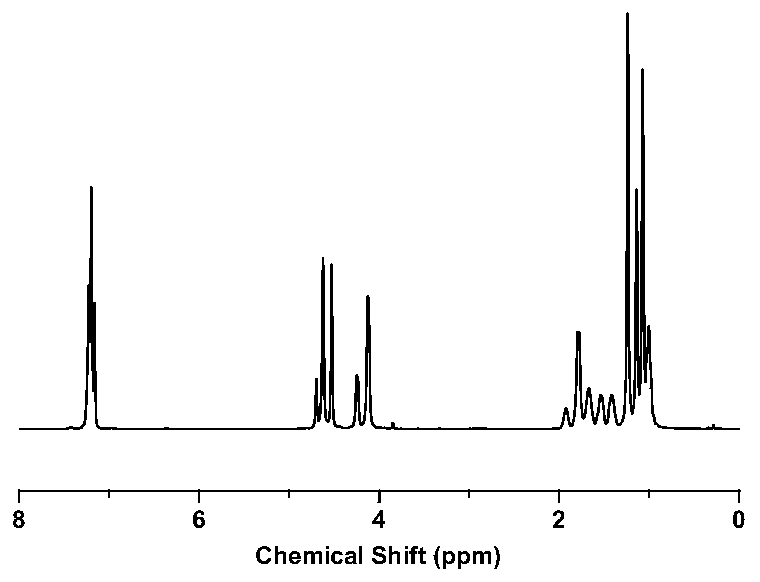
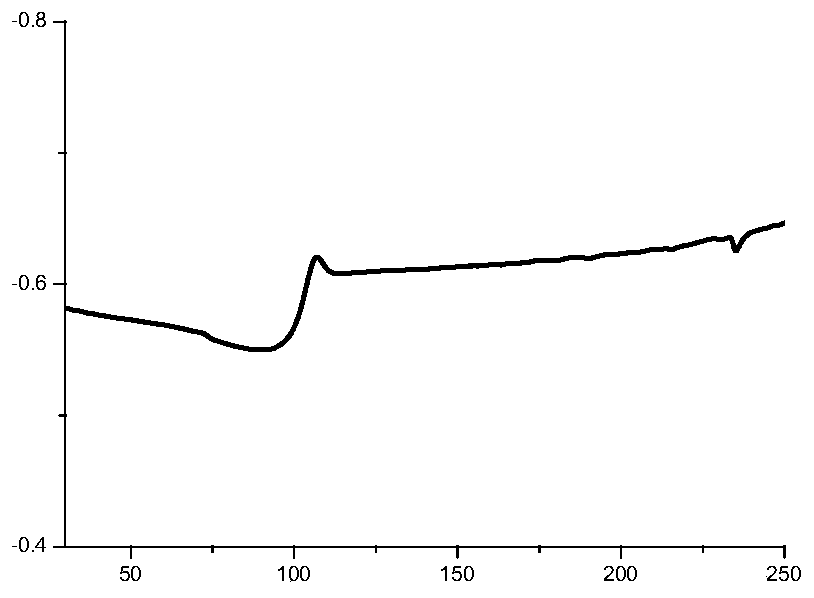
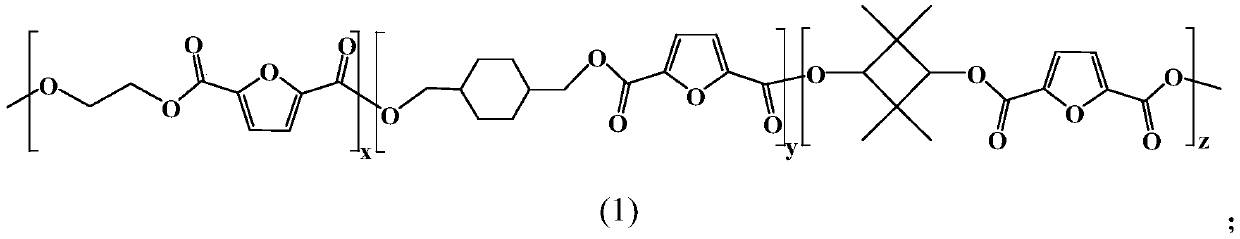
Furan dicarboxylic acid copolyester and preparation method thereof
A technology of furandicarboxylic acid copolyester and dibasic acid, applied in the field of materials, can solve problems such as precocious puberty, infant deformity, slow release of bisphenol A, etc., and achieve the effect of excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of furandicarboxylic acid copolyester provided by the invention may further comprise the steps:
[0032] (1) Dibasic acid or its esterified product, dibasic alcohol and esterification reaction catalyst are mixed, carry out esterification reaction under inert atmosphere, obtain the first intermediate product; Wherein, described dibasic acid comprises furandicarboxylic acid, so The dihydric alcohols include ethylene glycol, 1,4-cyclohexanedimethanol and 2,2,4,4-tetramethyl-1,3-cyclobutanedimethanol;
[0033] (2) Under vacuum conditions, subjecting the first intermediate product to a precondensation reaction to obtain a second intermediate product;
[0034] (3) Under vacuum conditions, the second intermediate product is subjected to polycondensation reaction to obtain furandicarboxylic acid copolyester.
[0035] Specifically, the furandicarboxylic acid is 2,5-furandicarboxylic acid, and the 2,5-furandicarboxylate is dimethyl 2,5-furandicarboxylate. ...
Embodiment 1
[0058] Dimethyl 2,5-furandicarboxylate 46g, 1,4-cyclohexanedimethanol 12.6g, 2,2,4,4-tetramethyl-1,3-cyclobutanediol 28.8g and ethyl 7.0 g of diol was added to the polymerization reactor, and then 0.15% of anhydrous zinc acetate based on the molar amount of dimethyl 2,5-furandicarboxylate was added. Under an inert atmosphere, react at 180° C. for 4 hours to obtain the first intermediate product.
[0059] Add 0.2% antimony trioxide, 0.15% triphenyl phosphate and 0.1% antioxidant 1010 based on the molar weight of dimethyl 2,5-furandicarboxylate to the first intermediate product, at a vacuum degree of 500Pa ~3000Pa, 220°C pre-condensation for 0.5h to obtain the second intermediate product.
[0060] Then the second intermediate product was reacted at 240° C. for 3 hours under a vacuum of 200 Pa to obtain furandicarboxylic acid copolyester.
[0061] After testing, the relative number average molecular mass of the furandicarboxylic acid copolyester is 23000g / moL, the relative weig...
Embodiment 2
[0065] Dimethyl 2,5-furandicarboxylate 92.5g, 1,4-cyclohexanedimethanol 54.1g, 2,2,4,4-tetramethyl-1,3-cyclobutanediol 10.0g and 22.0 g of ethylene glycol was added to the polymerization reactor, and then 0.10% of anhydrous zinc acetate based on the molar amount of dimethyl 2,5-furandicarboxylate was added. Under an inert atmosphere, react at 160° C. for 2 h to obtain the first intermediate product.
[0066] Add 0.1% antimony trioxide, 0.25% triphenylphosphine and 0.5% antioxidant 168 based on the molar weight of dimethyl 2,5-furandicarboxylate to the first intermediate product, at a vacuum degree of 500Pa ~3000Pa, 230°C precondensation for 1.0h, to obtain the second intermediate product.
[0067] Then the second intermediate product was reacted at 250° C. for 4 hours under a vacuum of 200 Pa to obtain furandicarboxylic acid copolyester.
[0068] After testing, the relative number average molecular mass of the furandicarboxylic acid copolyester is 36000g / moL, the relative we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative number average molecular mass | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com