Preparation method for diether diphthalimide
A technology of diphthalimide and phthalimide, which is applied in the field of preparation of diether diphthalimide, can solve problems such as uneven heating, high boiling point, solvent residue, etc. Simple, waste water reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
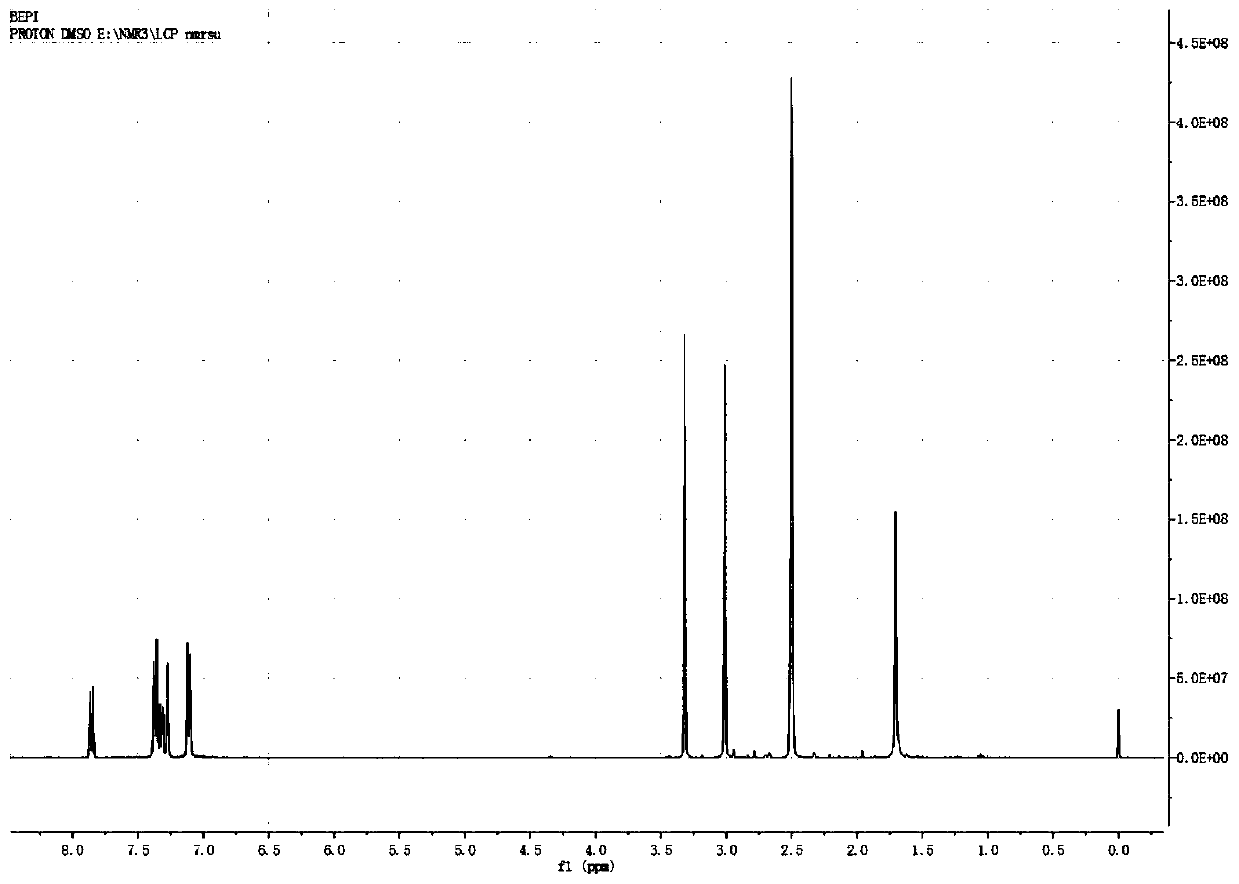
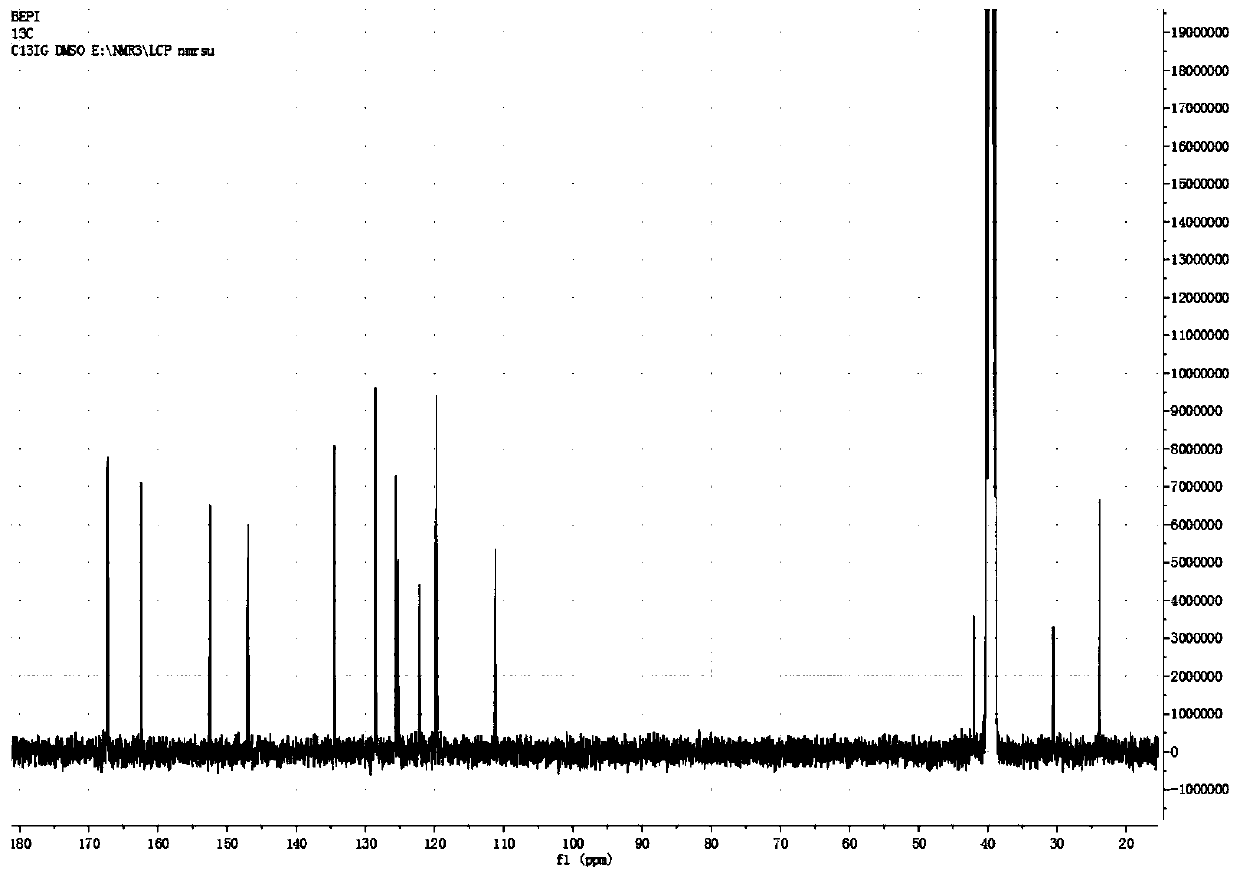
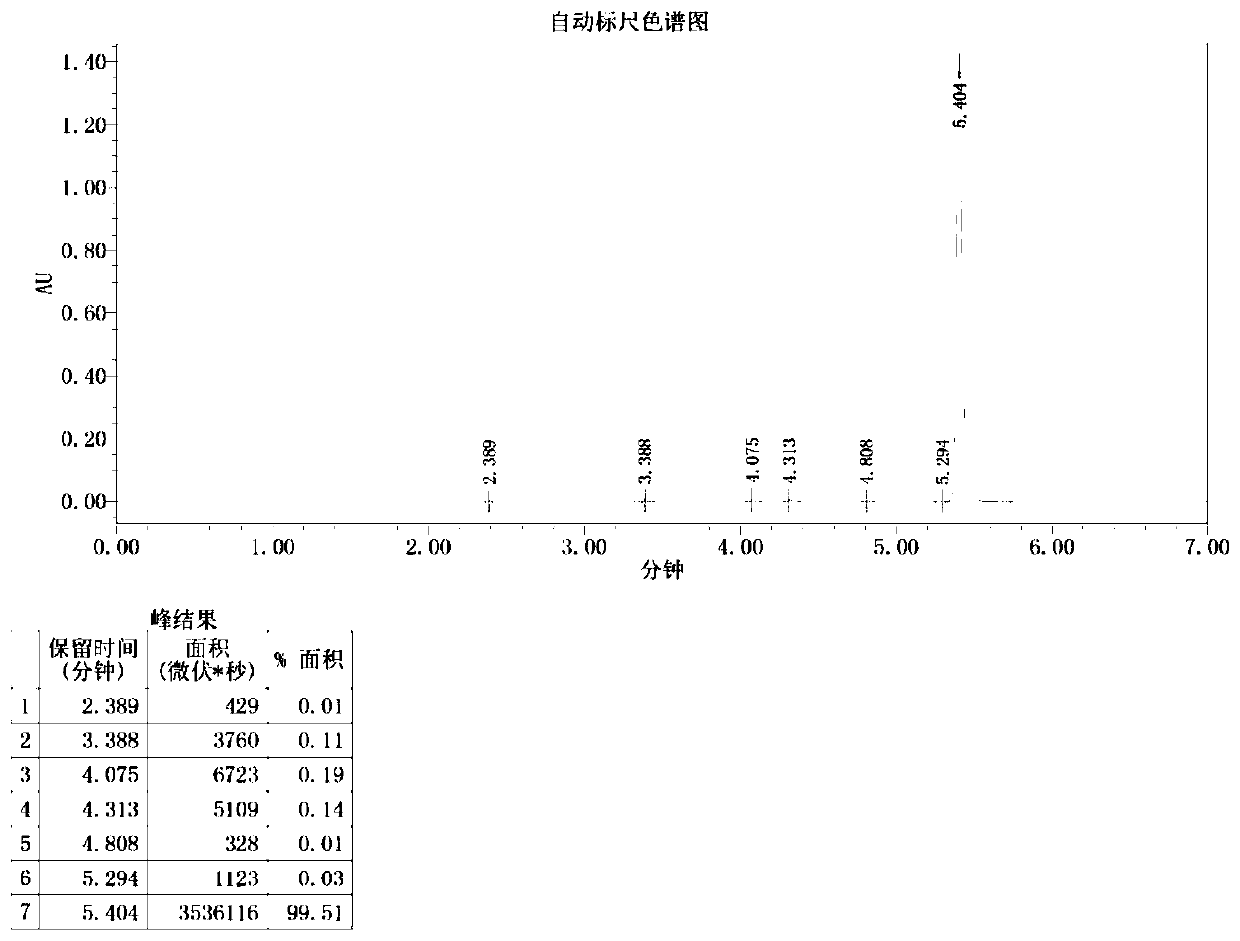
Image
Examples
Embodiment 1
[0047] Add 150 mL of o-xylene in a 500 mL three-necked flask, start stirring, feed nitrogen, then add bisphenol A (22.8 g, 0.1 mol) and sodium hydroxide (8.8 g, 0.22 mol) in sequence, start heating, and the temperature of the system rises to After 100°C, continue to keep warm for 2 hours, then raise the temperature to the reflux of the system (about 143°C), divide the water for 2-3 hours until there is no drop of water in the water separator, and then add 1-butyl-3-methylimidazole hexafluorophosphate ( [BMIM][PF 6 ], 1.42g, 5mmol, purchased from Hubei Yuancheng Saichuang Technology), N-methyl-4-nitrophthalimide (4-NPI, 41.2g, 0.2mol), add 37mL of phthalimide Toluene, heated to 140°C, reacted for 8h, then cooled to normal temperature, poured into 200mL water, heated to 70°C, and kept stirring for 30min, then left to separate layers, took the upper o-xylene layer, repeatedly added water to wash three times, collected organic layer, evaporated to dryness to obtain 52.3g of dieth...
Embodiment 2
[0049] Add 150 mL of o-xylene in a 500 mL three-necked flask, start stirring, feed nitrogen, then add bisphenol A (22.8 g, 0.1 mol) and sodium hydroxide (8.8 g, 0.22 mol) in sequence, start heating, and the temperature of the system rises to After 100°C, continue to keep warm for 2 hours, then raise the temperature to reflux of the system (about 143°C), separate the water for 2-3 hours until there is no drop of water in the water separator, and then add 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF 4 ]), 1.13g, 5mmol, purchased from Bailingwei Technology Co., Ltd.), N-methyl-4-nitrophthalimide (4-NPI, 41.2g, 0.2mol), add 37mL o-xylene , heated to 140°C, reacted for 8h, then cooled to room temperature, poured into 200mL water, heated to 70°C, and kept stirring for 30min, then stood to separate layers, took the upper o-xylene layer, repeatedly added water to wash three times, and collected the organic layer and evaporated to dryness to obtain 51.8 g of diether diphth...
Embodiment 3
[0051] Add 300mL of toluene into a 1000mL three-necked flask, start the stirring, blow in nitrogen, then add bisphenol S (50.0g, 0.2mol) and potassium hydroxide (23.5g, 0.42mol) in sequence, turn on the heating, and the temperature of the system rises to 100°C Afterwards, continue to keep warm for 2 hours, then raise the temperature to the reflux of the system (about 110°C), separate the water for 3 to 4 hours until there is no drop of water in the water separator, and then add 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM ][PF 6 ], 4.5g, 16mmol), N-methyl-4-nitrophthalimide (4-NPI, 82.4g, 0.4mol), add 75mL of toluene, heat up to 110°C, react for 6h, then Cool to room temperature, pour into 400mL water, heat to 70°C, and keep stirring for 30min, then let stand to separate layers, take the upper toluene layer, add water to wash three times, collect the organic layer, and evaporate to dryness to obtain diether diphthalimide 107.1 g, yield 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com