Recycling method of negative electrode material of waste lithium ion battery
A technology for lithium ion batteries and negative electrode materials, which is applied in the field of recycling and utilization of negative electrode materials of waste lithium ion batteries, can solve the problems of aggravating dust pollution, greenhouse effect, waste of resources, etc., and achieves excellent electrochemical performance, saving resources, and simple steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
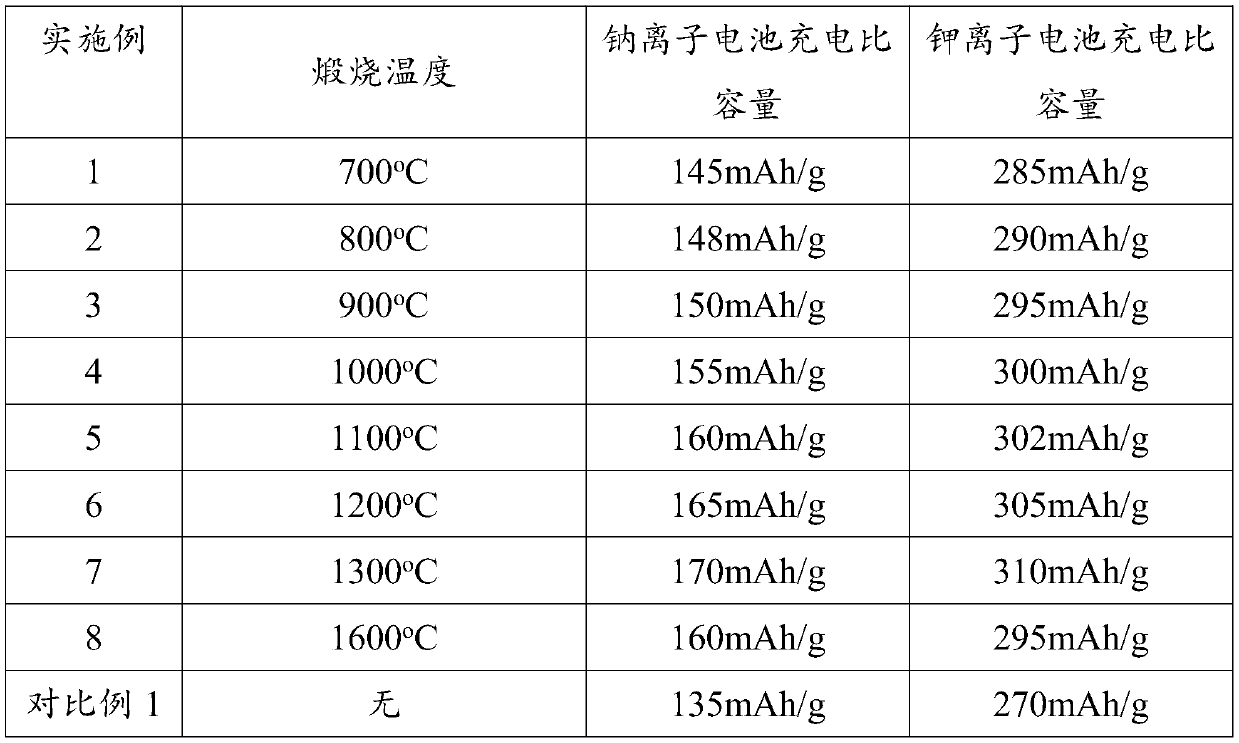
[0031] Disassemble the waste lithium cobalt oxide battery manually after discharging, use a utility knife to scrape off the positive electrode material on the positive electrode sheet, wash the scraped negative electrode material of the waste lithium ion battery with absolute ethanol for 3 times, centrifuge and dry to obtain Solid, the obtained solid material was put into a tube furnace under an argon atmosphere and calcined at 700° C. for 14 hours to obtain the final product recycled graphite.
[0032] The electrochemical performance of recycled graphite was tested using a half-cell:
[0033] Sodium ion battery: including positive pole piece, negative pole piece, diaphragm and electrolyte, wherein the active material of positive pole piece is recycled graphite, conductive agent is acetylene black, binder is polyvinylidene fluoride, recycled graphite, conductive agent and adhesive The mass ratio of the binder is 7:2:1, the sodium sheet is the counter electrode, the glass fiber...
Embodiment 2
[0039] Other conditions are the same as in Example 1, only the calcination temperature is changed to 800°C.
[0040] According to the method for embodiment 1, reclaim graphite is carried out electrochemical test, test result shows:
[0041] In sodium-ion batteries: the charge-discharge range is 0.01-2.8V, and the charge-discharge current density is 100mA / g, the material capacity can reach 148mAh / g, and the capacity has no obvious attenuation after 1000 cycles. Potassium ion battery: the charge and discharge range is 0.01-2V, and the charge and discharge at a current density of 50mA / g, the material capacity can reach 290mAh / g, and the capacity has no obvious attenuation after 100 cycles.
[0042] The above results prove that the method provided by the present invention can successfully recycle the graphite in the waste lithium cobalt oxide battery, and the final obtained material has higher capacity and stable cycle performance. The test results are listed in Table 1.
Embodiment 3
[0044] Other conditions are the same as in Example 1, only the calcination temperature is changed to 900°C.
[0045] According to the method for embodiment 1, reclaim graphite is carried out electrochemical test, test result shows:
[0046] In sodium-ion batteries: the charge-discharge range is 0.01-2.8V, and the charge-discharge current density is 100mA / g, the material capacity can reach 150mAh / g, and the capacity has no obvious attenuation after 1000 cycles. Potassium ion battery: the charge and discharge range is 0.01-2V, and the charge and discharge at a current density of 50mA / g, the material capacity can reach 290mAh / g, and the capacity has no obvious attenuation after 100 cycles.
[0047] The above results prove that the method provided by the present invention can successfully recycle the graphite in the waste lithium cobalt oxide battery, and the final obtained material has higher capacity and stable cycle performance. The test results are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com