Gliclazide sustained release tablet and preparation method thereof
A technology of gliclazide and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Blood sugar gastrointestinal problems, etc., to achieve rapid onset, strong lipid-lowering effect, and increase the effect of lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
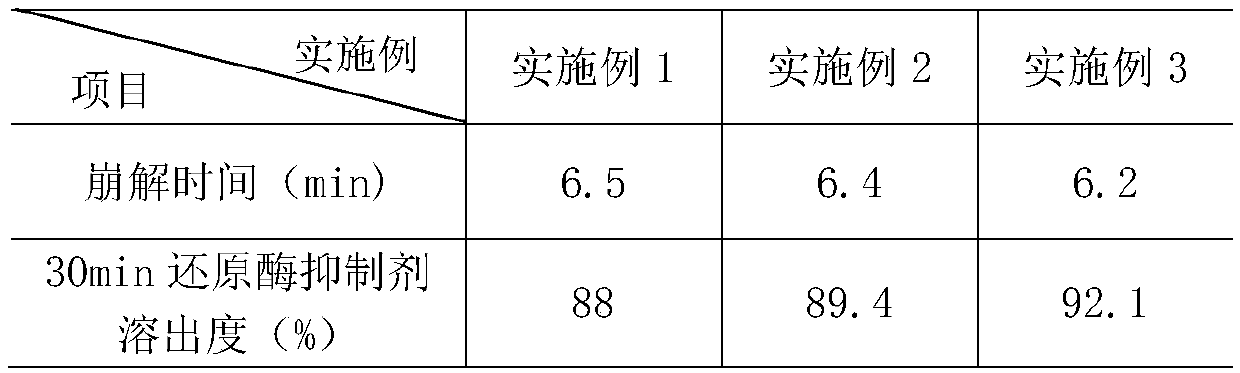
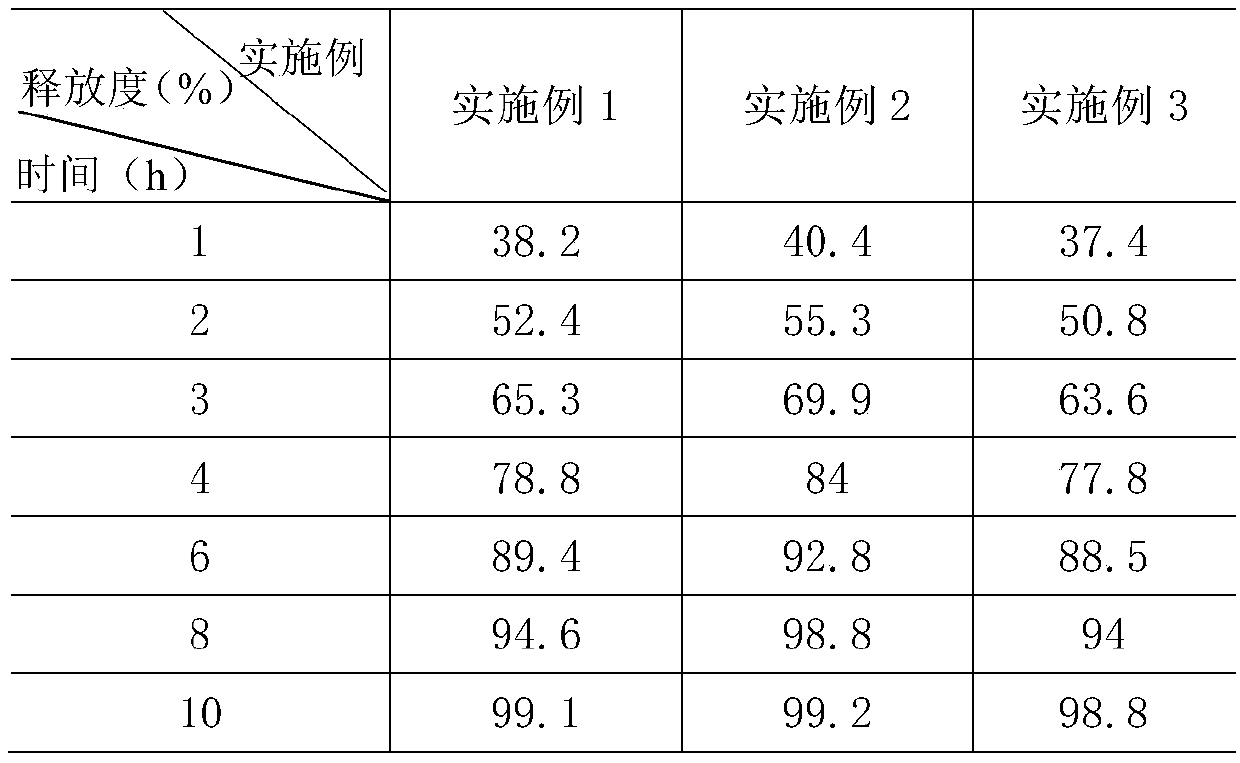
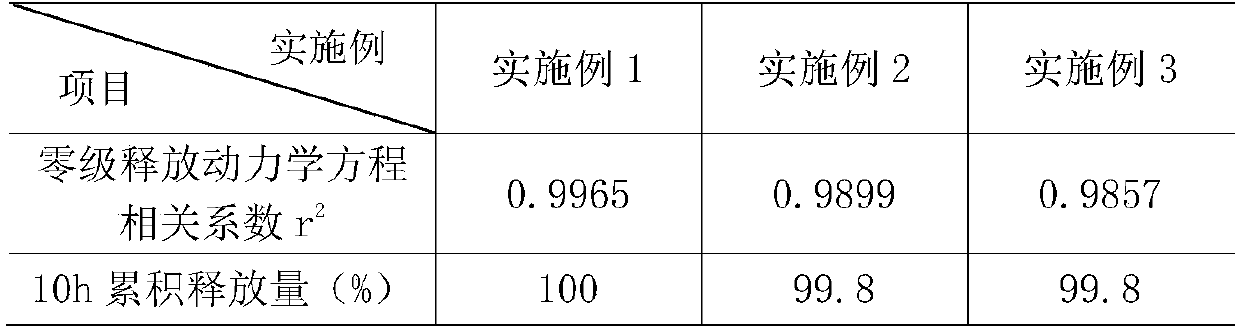
Embodiment 1
[0032] A gliclazide sustained-release tablet, consisting of a sustained-release layer and an auxiliary material layer with a weight ratio of 1.8:0.8:
[0033] The slow-release layer includes the following components by weight: 132 parts of gliclazide, 330 parts of skeleton material, 180 parts of filler for the slow-release layer, 6 parts of povidone, and 4.5 parts of camellia oil;
[0034] The auxiliary material layer includes the following components by weight: 42 parts of hydroxymethylglutaryl-CoA reductase inhibitor, 40.5 parts of montmorillonite, 30 parts of fruit and vegetable powder, 36 parts of microcrystalline cellulose, and 5.25 parts of vitamin C , 2.4 parts of deionized water, 3 parts of camellia oil.
[0035] The specific preparation method is as follows:
[0036] (1) Take each raw material respectively according to the above proportioning ratio, and set aside;
[0037] (2) Gliclazide, hydroxymethylglutaryl-CoA reductase inhibitor passed through 80-100 mesh sieve...
Embodiment 2
[0047]A gliclazide sustained-release tablet, consisting of a sustained-release layer and an auxiliary material layer with a weight ratio of 1.8:0.8:
[0048] The slow-release layer includes the following components by weight: 100 parts of gliclazide, 330 parts of skeleton material, 180 parts of filler for the slow-release layer, 6 parts of povidone, and 4.5 parts of camellia oil;
[0049] The auxiliary material layer includes the following components by weight: 30 parts of hydroxymethylglutaryl-CoA reductase inhibitor, 40.5 parts of montmorillonite, 30 parts of fruit and vegetable powder, 36 parts of microcrystalline cellulose, and 5.25 parts of vitamin C , 2.4 parts of deionized water, 3 parts of camellia oil.
[0050] The specific preparation method is as follows:
[0051] (1) Take each raw material respectively according to the above proportioning ratio, and set aside;
[0052] (2) Gliclazide, hydroxymethylglutaryl-CoA reductase inhibitor passed through 80-100 mesh sieve,...
Embodiment 3
[0062] A gliclazide sustained-release tablet, consisting of a sustained-release layer and an auxiliary material layer with a weight ratio of 2:0.6:
[0063] The slow-release layer includes the following components by weight: 132 parts of gliclazide, 330 parts of skeleton material, 180 parts of filler for the slow-release layer, 6 parts of povidone, and 4.5 parts of camellia oil;
[0064] The auxiliary material layer includes the following components by weight: 42 parts of hydroxymethylglutaryl-CoA reductase inhibitor, 40.5 parts of montmorillonite, 30 parts of fruit and vegetable powder, 36 parts of microcrystalline cellulose, and 5.25 parts of vitamin C , 2.4 parts of deionized water, 3 parts of camellia oil.
[0065] The specific preparation method is as follows:
[0066] (1) Take each raw material respectively according to the above proportioning ratio, and set aside;
[0067] (2) Gliclazide, hydroxymethylglutaryl-CoA reductase inhibitor passed through 80-100 mesh sieve, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com