Perovskite TiSrCo Catalyst for Hydrogen Production by Autothermal Reforming of Acetic Acid
A perovskite type, autothermal reforming technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as catalyst deactivation, and achieve Effects of reduced activation energy, stable activity, and high hydrogen yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Weigh 2.107g of Co(NO 3 ) 2 ·6H 2 O, add 30.0ml of deionized water, stir at room temperature for 30min to prepare #1 solution; then weigh 14.494g of C 16 h 36 o 4 Ti, add 3.0ml of hydrochloric acid (35%) and 9.3ml of anhydrous acetic acid, fully stir at room temperature for 30min to dissolve, and obtain #2 solution; then weigh 2.890g of P123 solution, add 30.0ml of absolute ethanol, Stir at room temperature for 30 minutes to prepare #3 solution; slowly drop #1 solution into #3 solution, stir for 30 minutes, then slowly drop the obtained solution into #2 solution, stir at 40°C for 3 hours, let stand, Gel forming; put the obtained gel into an oven at 65°C and dry for 48 hours; then bake the dried sample at 600-800°C for 4 hours, press into tablets, and sieve to obtain the catalyst CDUT-LHG-TC, The composition is TiCoO 3 . The weight percent composition of the catalyst is: 15.0% of cobalt oxide and 85.0% of titanium dioxide.
[0030] The evaluation of autothermal r...
Embodiment 1
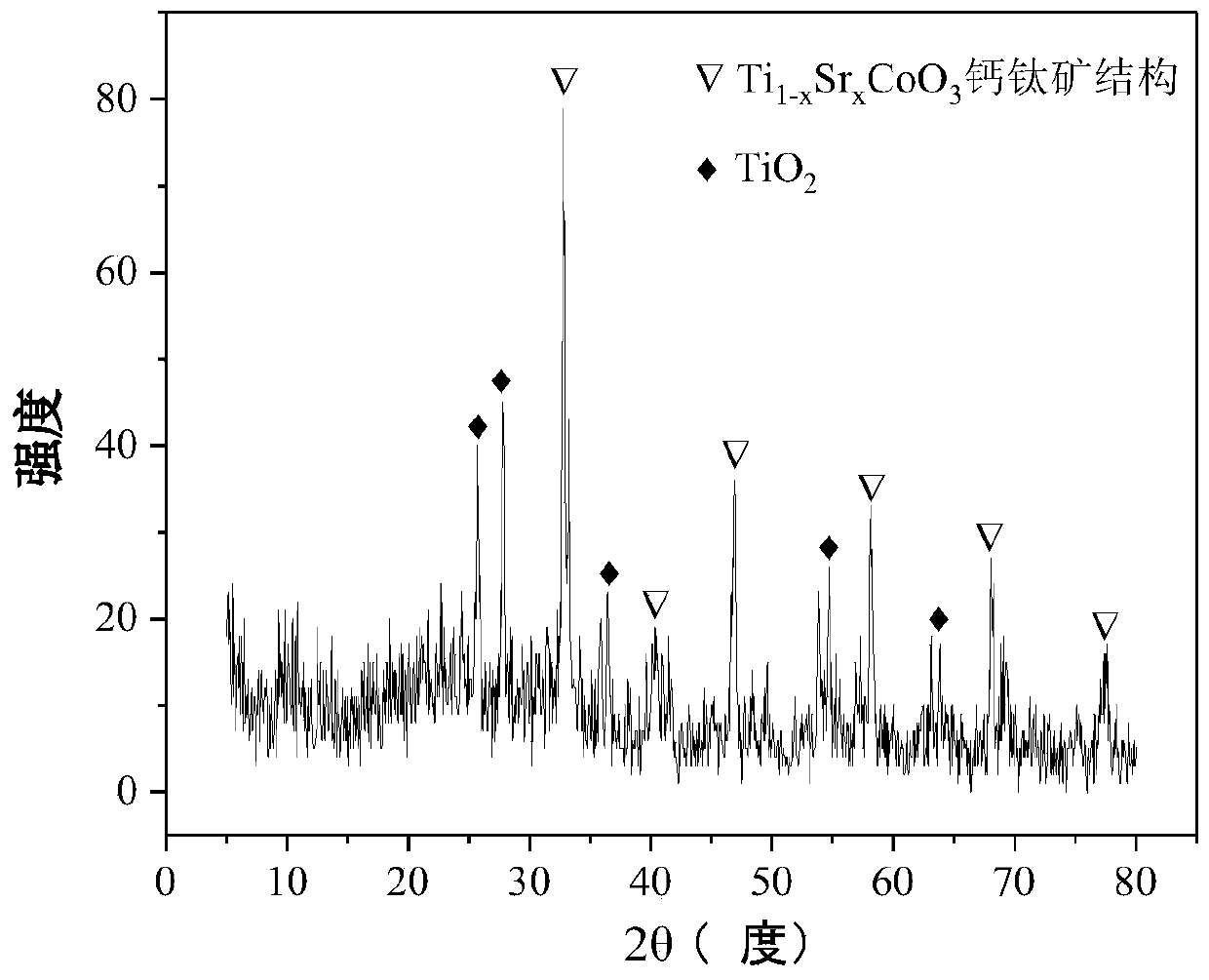
[0033] Weigh 2.106g of Co(NO 3 ) 2 ·6H 2 O and 1.701 g of SrNO 3 , add 30.0ml of deionized water, stir at room temperature for 30min to prepare #1 solution; then weigh 10.945g of C 16 h 36 o 4 Ti, add 2.3ml of hydrochloric acid (35%) and 7.0ml of anhydrous acetic acid, fully stir at room temperature for 30min to dissolve, and obtain #2 solution; then weigh 2.751g of P123 solution, add 30.0ml of absolute ethanol, Stir at room temperature for 30 minutes to prepare #3 solution. Subsequent steps are the same as in reference example 1 to obtain catalyst CDUT-LHG-TSC-1, the composition of which is Ti 0.8 Sr 0.2 CoO 3 , its XRD pattern is attached figure 1 , indicating that the catalyst formed a typical perovskite structure and a small amount of TiO 2 . The weight percent composition of the catalyst is: 15.0% of cobalt oxide, 64.2% of titanium dioxide and 20.8% of strontium oxide.
[0034] The CDUT-LHG-TSC-1 catalyst was investigated for the activity of autothermal reform...
Embodiment 2
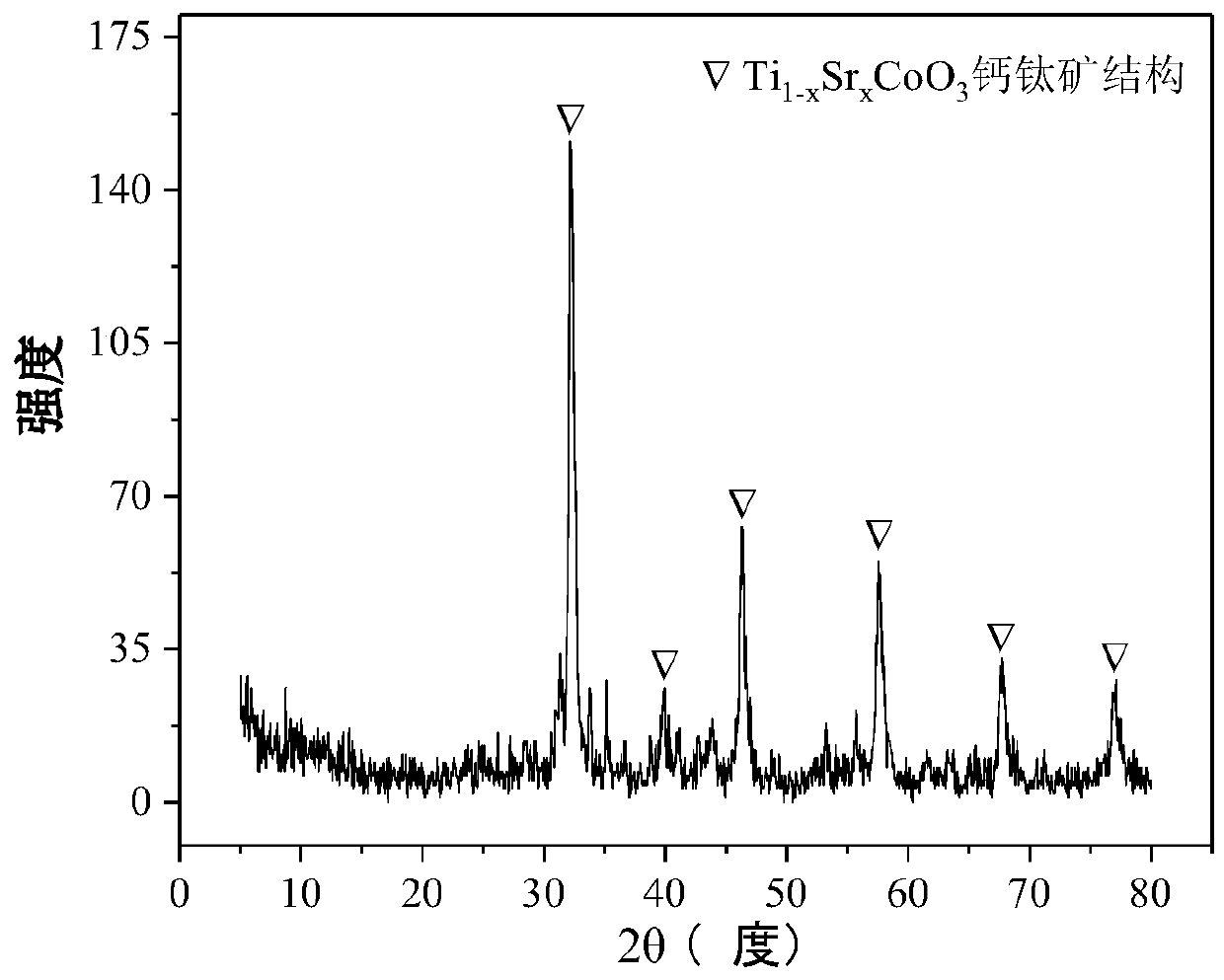
[0036] Weigh 2.108g of Co(NO 3 ) 2 ·6H 2 O and 3.918g of SrNO 3 , add 30.0ml of deionized water, stir at room temperature for 30min to prepare #1 solution; then weigh 6.302g of C 16 h 36 o 4 Ti, add 1.48ml of hydrochloric acid (35%) and 4.6ml of anhydrous acetic acid, fully stir at room temperature for 30min to dissolve, and obtain #2 solution; then weigh 2.930g of P123 solution, add 30.0ml of absolute ethanol, Stir at room temperature for 30 minutes to prepare #3 solution. Subsequent steps are with reference example 1, obtain catalyst CDUT-LHG-TSC-2, and its composition is Ti 0.5 Sr 0.5 CoO 3 , and its typical XRD pattern is attached figure 2 , indicating that the catalyst formed a typical perovskite structure Ti 1-x Sr x CoO 3 , The weight percent composition of the catalyst is: cobalt oxide 15.0%, titanium dioxide 37.0%, strontium oxide 48.0%.
[0037] The CDUT-LHG-TSC-2 catalyst was investigated for the activity of autothermal reforming of acetic acid. The re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com