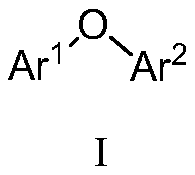
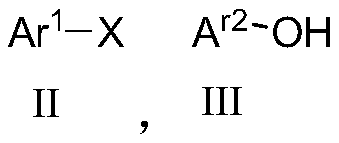Preparation method of diaryl ether compound
A compound and aryl technology, applied in the field of preparation of diaryl ether compounds, can solve the problems of generating solid waste, toxicity, corrosion, etc., and achieve the effects of zero discharge of three wastes, mild reaction conditions, and production cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] In a 50ml reaction bottle, add 0.1g of copper oxide nanoparticles, 1g of chitosan and 20ml of toluene, sonicate to obtain a uniform suspension, and stir at room temperature to ensure that the chitosan surface adsorbs enough Cu2O nanoparticles, filter, Washed with ethanol and dried under vacuum at 50 °C to obtain CS-supported Cu 2 O(CS@Cu 2 O). Refer to this method to prepare CS@CuSO 4 , CS@CuI and CS@Cu(OAc) 2 to spare.
Embodiment 2
[0052]
[0053] Add bromobenzene, phenol, catalyst, alkali and solution to the reaction in sequence, heat and stir for 18 hours, add water to quench the reaction, filter, use for ethyl acetate extraction, wash with saturated brine, dry, concentrate, and perform silica gel column chromatography in diphenyl ether. To investigate the catalyst effectiveness of different reaction conditions, the specific data are as described in Table 1:
[0054] Table 1
[0055]
[0056]
[0057] a General reaction conditions: 1.5mmol phenol, 1.0mmol bromobenzene, 2.0mmol potassium phosphate, 1mL of anhydrous solvent; b3.0mmol potassium phosphate; c 2mmol phenol.
[0058] Conclusion: Using the coupling reaction of bromobenzene and phenol as a template, the catalytic effects of different catalysts (A, B, C, D) were preliminarily evaluated. When 0.01 equivalent of [Cu] and 2 equivalents of K are present in DMF 3 PO 4 When , the coupling reaction of p-bromobenzene and phenol showed good ...
Embodiment 3
[0060]
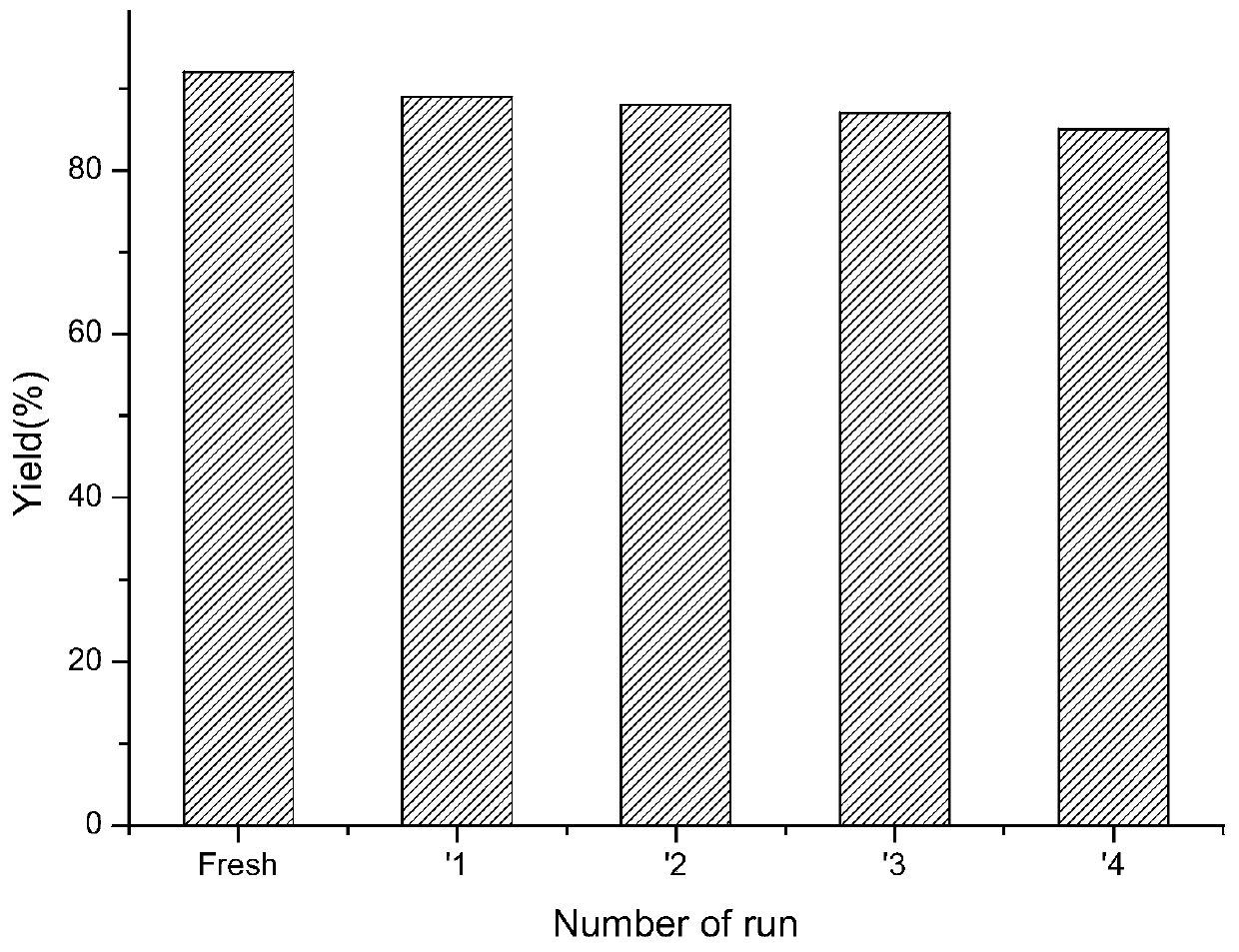
[0061] Add 1mmol formula II compound, 2mmol formula III compound, 0.5mmol% catalyst CS@Cu to the reaction successively 2 O. 3mmol potassium phosphate and 0.5ml N,N-dimethylformamide solution, heated to 110°C and stirred for reaction. After the reaction was detected by TLC, add water to quench the reaction, filter, use for ethyl acetate extraction, and wash with saturated saline , dried, concentrated, and silica gel column chromatography to obtain diphenyl ether.
[0062]
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com