Preparation method and application of total flavonoids from resina draconis
A technology of total flavonoids of dragon's blood and dragon's blood, which is applied in the field of medicine, can solve the problems of easy elution, complex components, toxic and side effects, etc., and achieve the effect of long-lasting drug effect and prevention and treatment of atherosclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment provides a preparation method of total flavonoids of dragon's blood, the specific process is as follows:
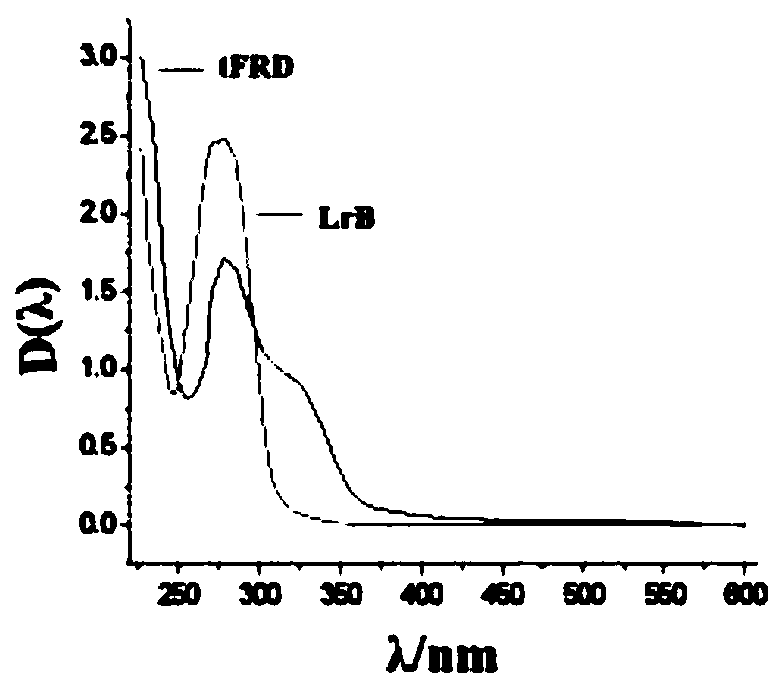
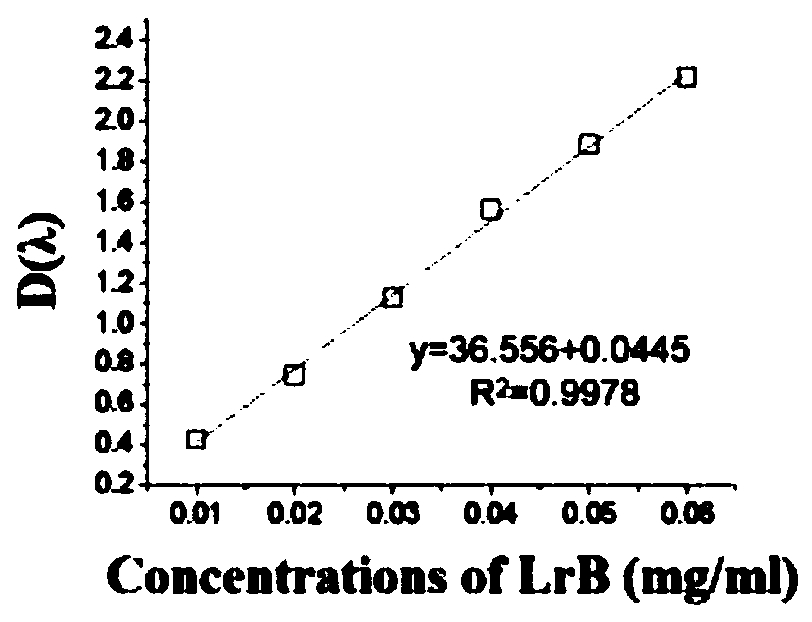
[0028] Crush the medicinal material of dragon's blood into a coarse powder, add 11 times the amount of ethanol and ethyl acetate mixed solvent, and reflux extraction at 50-69°C for 2 times, each time for 15-29 minutes; among them, ethanol and ethyl acetate The volume ratio of ester is 5:5~1:10. Preferably, the reflux extraction temperature is 65° C., each reflux extraction time is 25 minutes, and the volume ratio of ethanol to ethyl acetate is 2:8. The content of total flavonoids in the extract was taken as reference substance with loureirin B (Loureirin B, LrB, Shanghai Chunyou Biotechnology Co., Ltd.), and the absorbance of the reference substance and the extract was measured at 277nm by a UV spectrophotometer, and the concentration of the reference substance was taken as the horizontal Coordinates, the absorbance value is the ordinate, draws a s...
Embodiment 2
[0033] In this embodiment, the up-and-down method is used to measure the total flavonoids of dragon's blood and the acute toxicity of dragon's blood. The specific measurement process is as follows: select two groups of female mice, A and B, with five mice in each group, and treat the mice in group A with 2000 mg according to the up-down method. The total flavonoids of dragon's blood was injected intraperitoneally at the dose level of 2000 mg / kg, and the mice in group B were injected with dragon's blood at a dose level of 2000 mg / kg intraperitoneally, and the survival of the mice in the two groups was observed. Among them, the operation method of the upper and lower method, the limit test of the dose level of 2000mg / kg: the test substance is given to a mouse respectively, if the mouse dies, then the main test is carried out; if the mouse survives, the two drugs are given in sequence Give another 4 mice separately, and the total number of animals is 5; if one animal dies in the l...
Embodiment 3
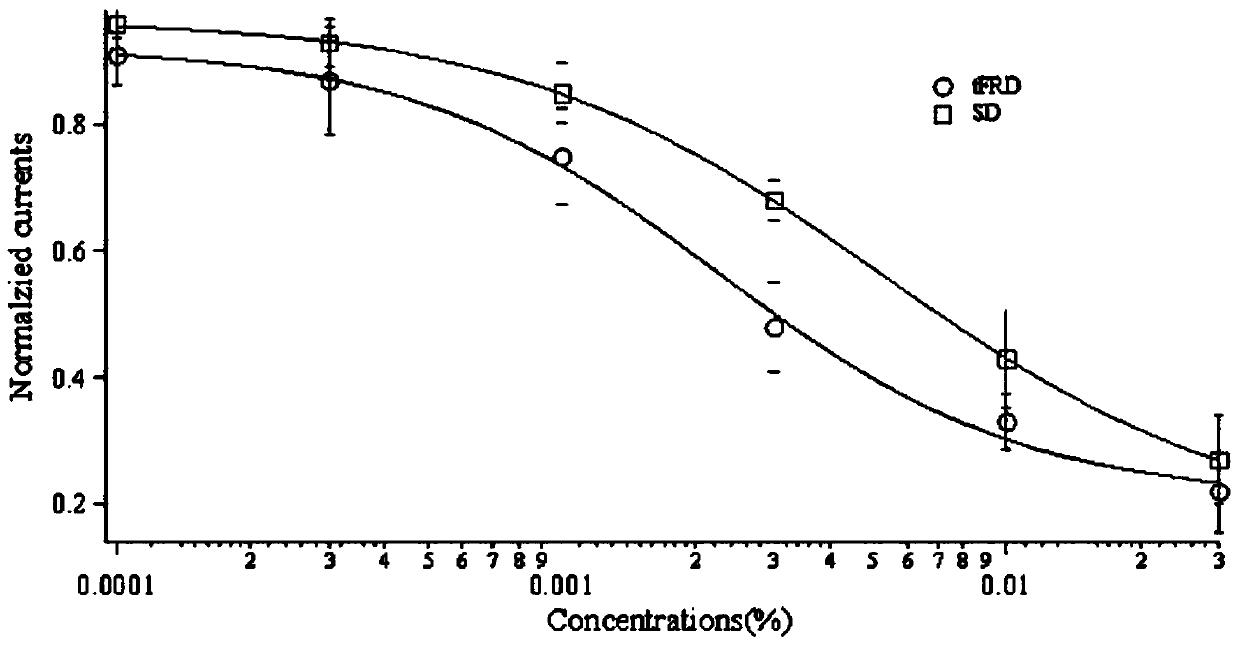
[0036] In this embodiment, the patch clamp experiment is used to detect the inhibitory effect of the total flavonoids of dragon's blood on the Kv1.3 channel function of mammals. The specific process is as follows:
[0037] Human leukemia lymphocyte line Jurkat T cells and HEK-293T cells (Wuhan University Collection Center) were cultured in RPMI 1640 ( Hyclone) and high-glucose DMEM (Hyclone) medium, at 37°C, 5% CO 2 cultured in an incubator. The eukaryotic expression vector pIRES2-EGFP (provided by the laboratory of Professor Li Wenxin of Wuhan University) encoding voltage-gated potassium channels Kv1.1, Kv1.2 and Kv1.3 was transfected into HEK-293T with lipofectamine 2000 (Invitrogen) In cells, green fluorescent cells were selected for electrophysiological tests after 24 hours. The composition of extracellular fluid (mmol / L) used to record voltage-gated potassium channel current in the experiment is: 140NaCl, 5KCl, 2CaCl 2 ,1MgCl 2 , 10HEPES, 10D-Glucose, the pH value of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com