Near infrared fluorescent probe capable of being used for responding to pH change in living cells in chronic wound development process and preparation method of probe
A fluorescent probe, chronic wound technology, applied in chemical instruments and methods, preparations for in vivo experiments, luminescent materials, etc. Fluorescence interference, avoidance of self-absorption, excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis routes of fluorescent probes 1, 2, and 3 that can be used for fluorescence imaging of exogenous pH changes in living cells are shown in the following reaction formula:
[0041]
[0042] Note: The structure of IR-780 is
[0043] (1) Synthesis of compound 1a
[0044] 2 g (13.1 mmol) of methyl 3-hydroxybenzoate were placed in a round bottom flask and dissolved in 8 mL of carbon tetrachloride. Then 0.7 mL (27.3 mmol) of bromine was slowly added dropwise at 0°C, and stirring was continued overnight at 50°C. After the reaction was completed, the reaction liquid was cooled at 0° C. for 2 h and then suction filtered. The crude product of the filter cake was recrystallized with carbon tetrachloride to obtain 2.1 g of white crystalline solid (1a) with a yield of 69%.
[0045] 1 H NMR (400MHz, DMSO-d 6 )δ10.12(s,1H),7.50(d,J=8.7Hz,1H),7.15(d,J=3.0Hz,1H),6.89(dd,J=8.7,3.0Hz,1H),3.83( s,3H). 13 C NMR (101MHz, DMSO-d 6 )δ166.49, 157.21, 135.21, 133.34, 120.7...
Embodiment 2
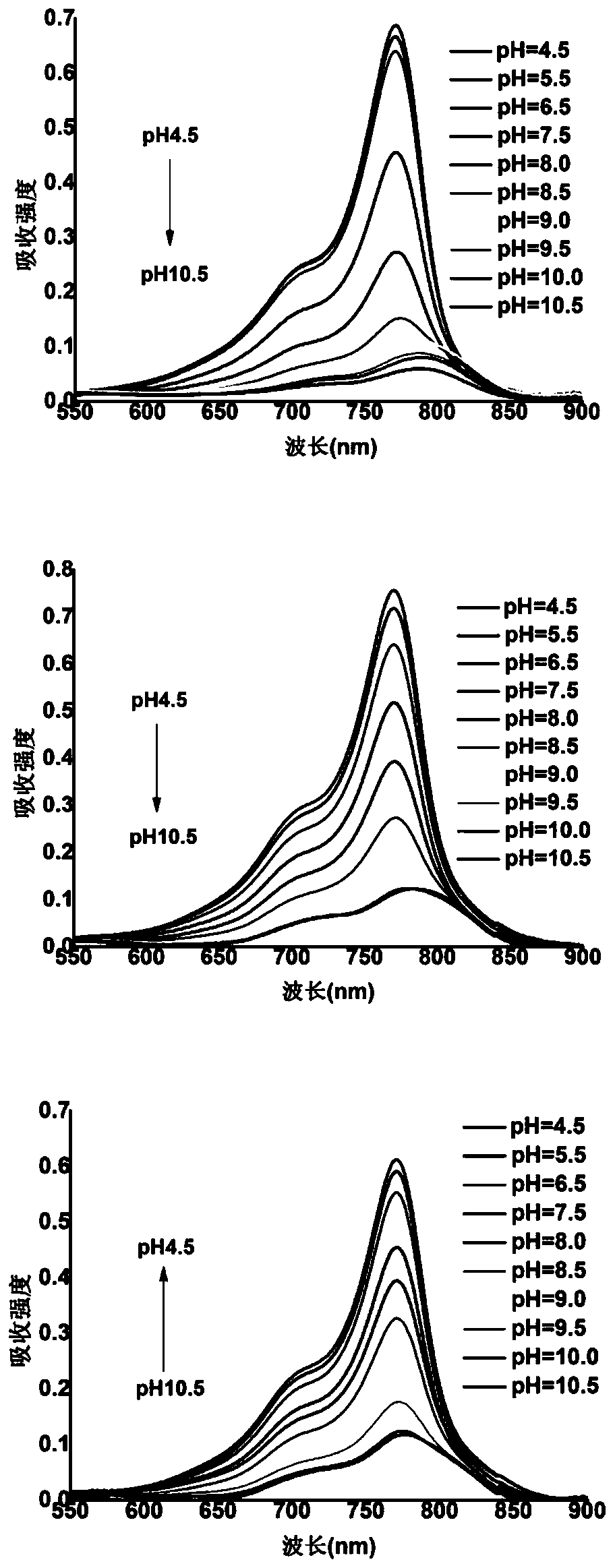
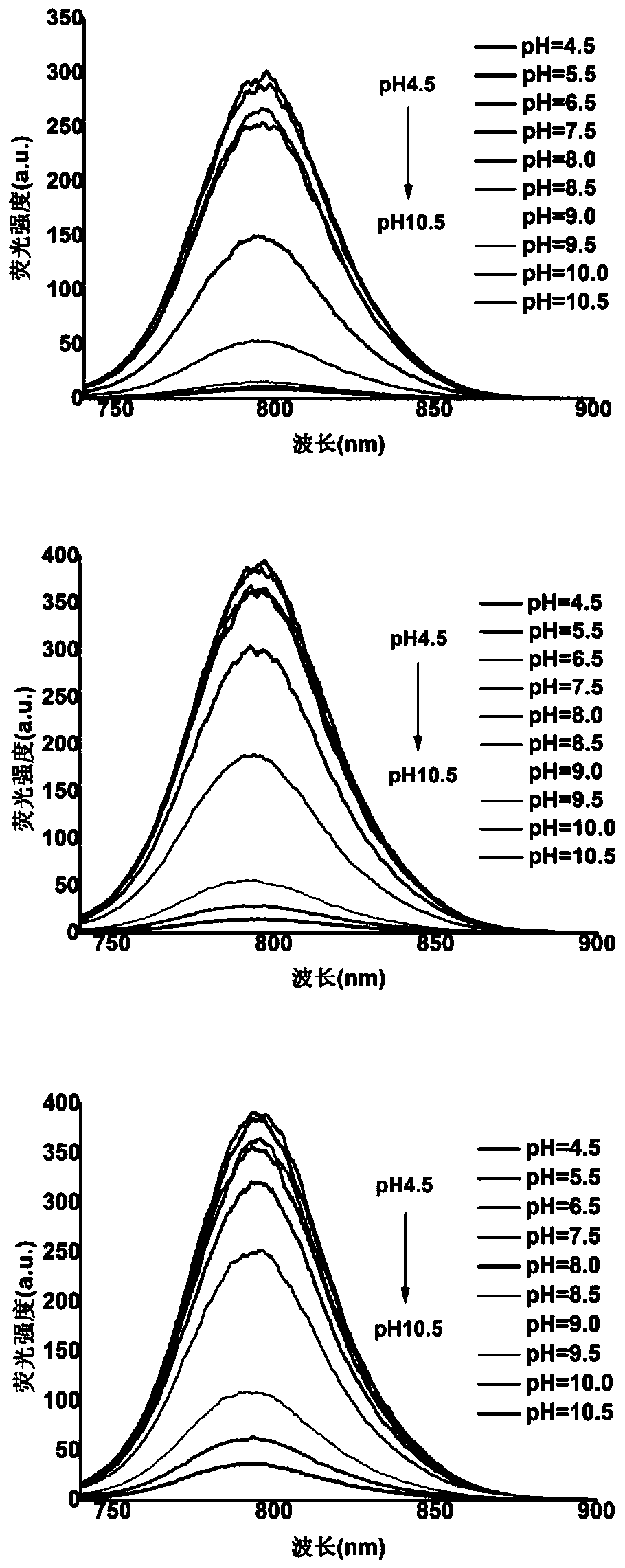
[0067] Example 2 Fluorescent Probes 1, 2, 3 Changes in UV Absorption Intensity and Fluorescence Intensity in Different pH Buffers
[0068] The prepared probes 1, 2, and 3 (10 μM) were placed in different buffers with a pH of 4.5-10.5 (phosphate-citric acid buffer for pH 4.5-pH 8.5, bicarbonate buffer for pH 9.0-pH 10.5) Salt buffer solution, all containing 10% DMSO), and test the ultraviolet absorption spectrum and fluorescence spectrum of the three, the results are as follows figure 1 and figure 2 As shown, the maximum absorption wavelength and maximum emission wavelength of probes 1-3 are 770nm and 798nm, respectively. Under acidic conditions, the nitrogen atom on the hydrazide group of the probe is protonated, and the photoinduced electron transfer (PET) is inhibited, so the three probes all reach the maximum fluorescence intensity at this time. As the pH increases, since the protonation of the nitrogen atom disappears, the PET effect of the probe itself recovers, result...
Embodiment 3
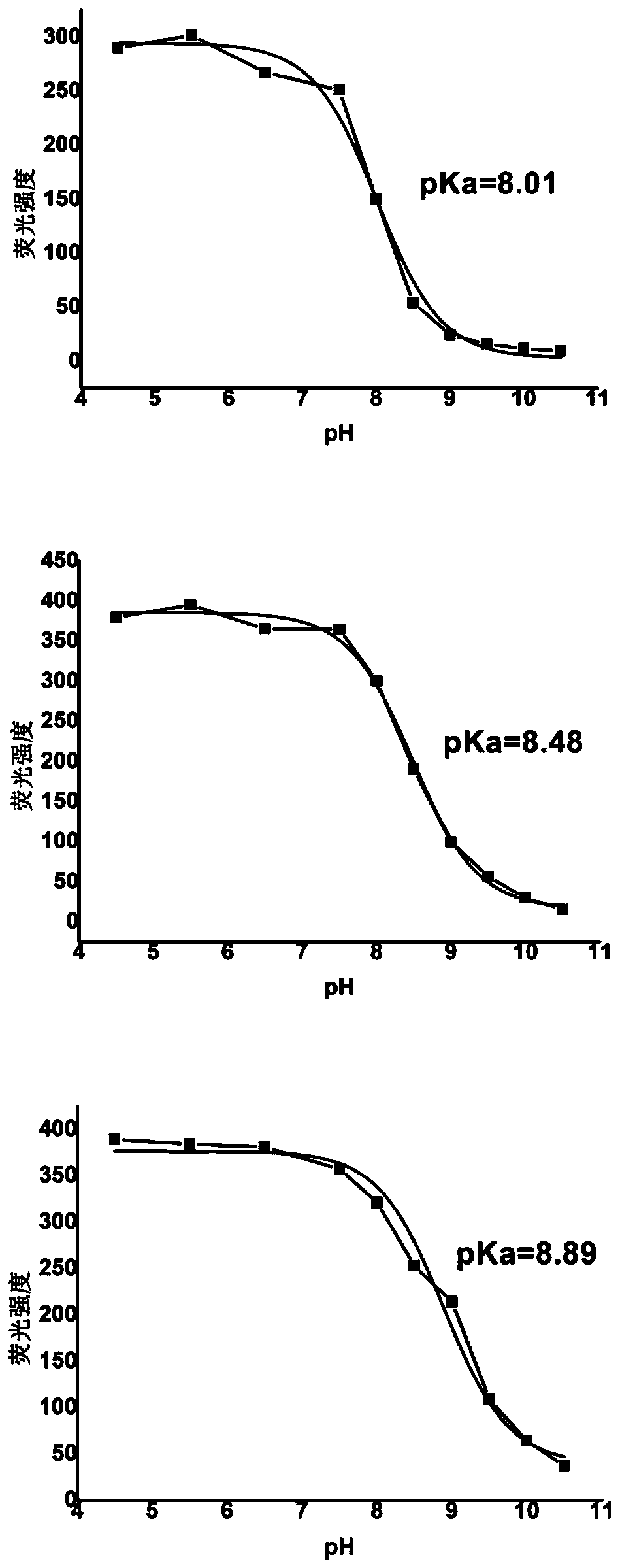
[0069] The pKa value contrast situation of embodiment 3 fluorescent probe 1,2,3
[0070] Fluorescence intensity changes at different pH values were measured by fluorescence titration experiments (the working concentration of the probe was 10 μM, and the excitation wavelength of the test was 715 nm), and the nonlinear curves of the three probes were fitted using Origin to obtain the pKa of the three probes Condition. As shown in Figure 3, the three probes all have a relatively obvious pH response mutation range, which is very important for finely measuring small fluctuations in pH. In addition, probe 2 without any substituents on the benzene ring has a pKa value of 8.48; when a bromine atom with a certain electron-withdrawing ability is added to the benzene ring (such as probe 1), its pKa value decreases to 8.01; and After adding a methoxy group with a certain electron-donating ability to the benzene ring (such as probe 3), its pKa value increases to 8.89. The above shows th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com