Alkylaromatic conversion catalyst
A catalyst, alkyl aromatic technology, applied in the field of alkyl aromatic hydrocarbon conversion catalysts, can solve problems such as affecting catalyst performance, expensive procedures, complexity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 (manufacture of catalyst A)
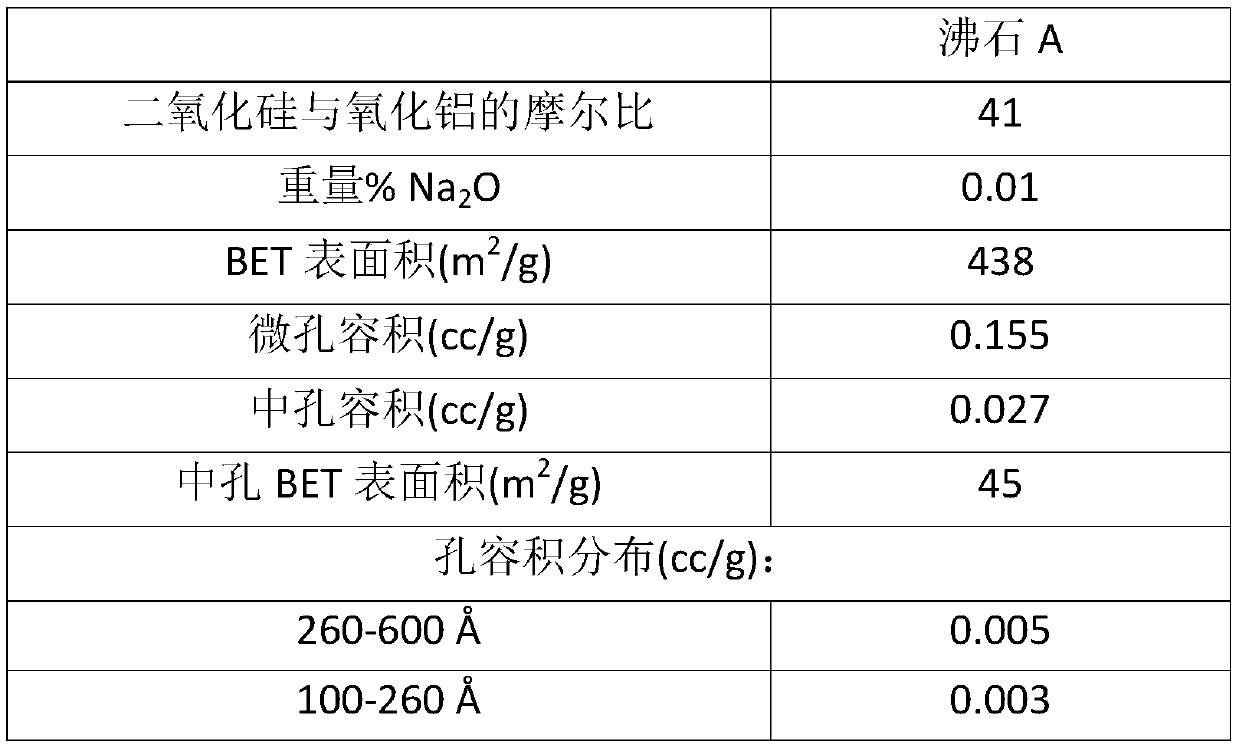
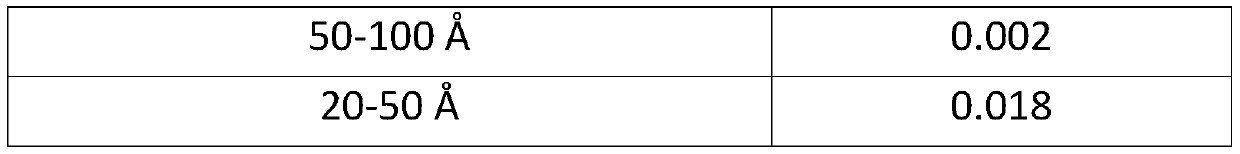
[0069] 92 g of solid sodium hydroxide and 125 g of L-tartaric acid were dissolved in 3.5 l of water, and 175 g of sodium aluminate solution was added thereto to prepare a uniform solution. Then, 660 g of silicic acid powder was slowly added to the mixed solution with stirring to prepare a uniform aqueous reaction mixture. The reaction mixture was placed in an autoclave, and after the autoclave was closed, it was allowed to react at 160° C. for 72 hours with stirring. Thereafter, the reaction product was taken out of the autoclave, washed with distilled water until its pH was almost neutral, then filtered and dried overnight at 120 °C. The product thus obtained is ZSM-5, the properties of which are shown in Table 1 below. This zeolite is hereinafter referred to as zeolite A.
[0070] Table 1
[0071]
[0072]
[0073] Micropore and mesopore volumes are each deduced from nitrogen adsorption and desorption isotherms ...
Embodiment 2
[0077] Embodiment 2 (manufacture of catalyst B)
[0078] A sample of Support A as described in Example 1 was dealuminated by treating the sample with a 0.02M aqueous solution of ammonium hexafluorosilicate. The samples thus treated were subsequently washed, dried and calcined.
[0079] The support thus obtained was impregnated in the pore volume with a solution containing platinum (but without tin) to obtain a final catalyst with 0.025 wt.% platinum based on the total catalyst. After impregnation is complete, the catalyst is dried and then calcined. The resulting catalyst is referred to as Catalyst B hereinafter.
Embodiment 3
[0080] Embodiment 3 (manufacture of catalyst C)
[0081] The support was prepared from ZSM-5 with a mesopore volume of 0.29 ml / g, a number average crystallite size of 28 nm and a silica to alumina molar ratio of 80. This ZSM-5 is commercially available from Zeolyst as "ZD13008", hereinafter referred to as Zeolite C. The high and medium pore volume in the zeolite translates to the high and medium pore volume of the zeolite in the catalyst.
[0082] Zeolite powder was mixed with low sodium grade silica ("Sipernat 50" from Degussa) and ammonium stabilized commercial silica sol (sold under the trade name "Bindzil 30NH3" by the company Eka Chemicals) and extruded To obtain a support comprising 60 wt.% of zeolite, 26.6 wt.% of "Sipernat 50" and 13.4 wt.% of silica sol on a dry basis.
[0083] The raw extrudates were dried and calcined at 500°C to achieve sufficient strength for industrial applications. The obtained carrier is hereinafter referred to as carrier C.
[0084] The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com