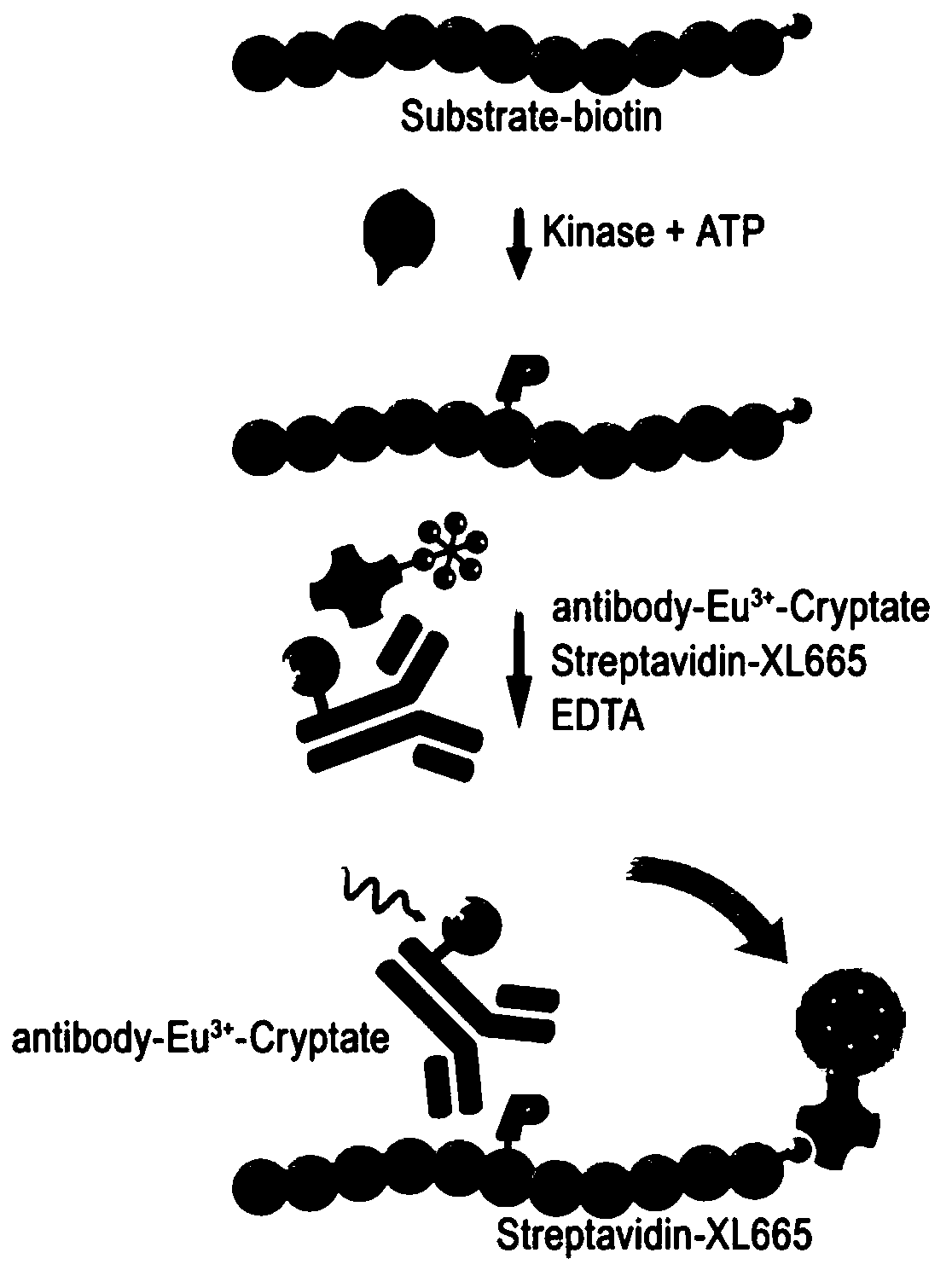
Method for screening kinase inhibitor by utilizing HTRF one-step method
A kinase inhibitor and one-step technology, which is applied in the field of one-step screening of kinase inhibitors using HTRF, can solve the problems of high-throughput or ultra-high-throughput screening of difficult compounds, experimental errors, and time-consuming, and achieve economical and reliable screening results. , The effect of saving experimental workload and saving experimental time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
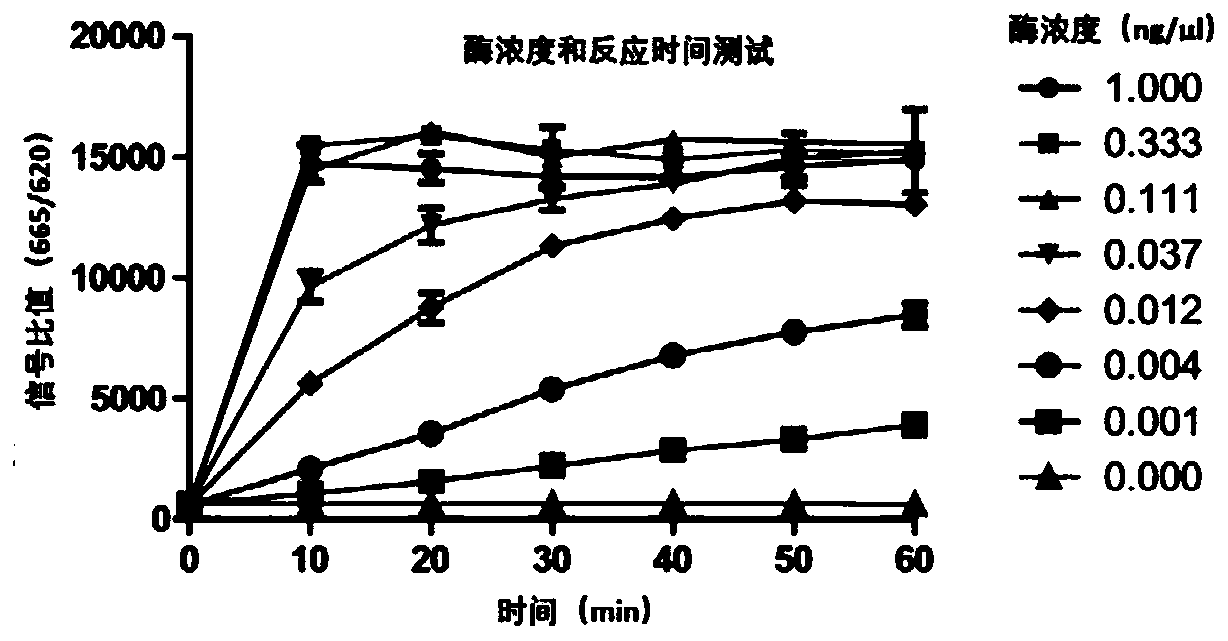
[0040] 1) To prepare EGFR kinase, start with 3-fold serial dilution from 1ng / ul. The substrate concentration is fixed at 1uM, the ATP concentration is 100uM, and the enzyme reaction time is 10, 20, 30, 40, 50, 60 minutes respectively;
[0041] 2) Add the prepared kinase, substrate, and ATP into the 384-well detection plate, incubate at room temperature for a corresponding period of time, add the detection reagent coupled with Donor and Acceptor (or add 10ul after equal volume mixing), incubate for 1 hour and read ;
[0042] According to the principle of HTRF method, the fluorescence ratio of 665nm / 620nm is detected. According to this ratio, the phosphorylation of the substrate is judged. The higher the signal, the higher the phosphorylation degree of the substrate, and the higher the kinase activity. The results are shown in figure 2 . figure 2 The Kd value was determined by MCL1 and BAK binding test.
Embodiment 2
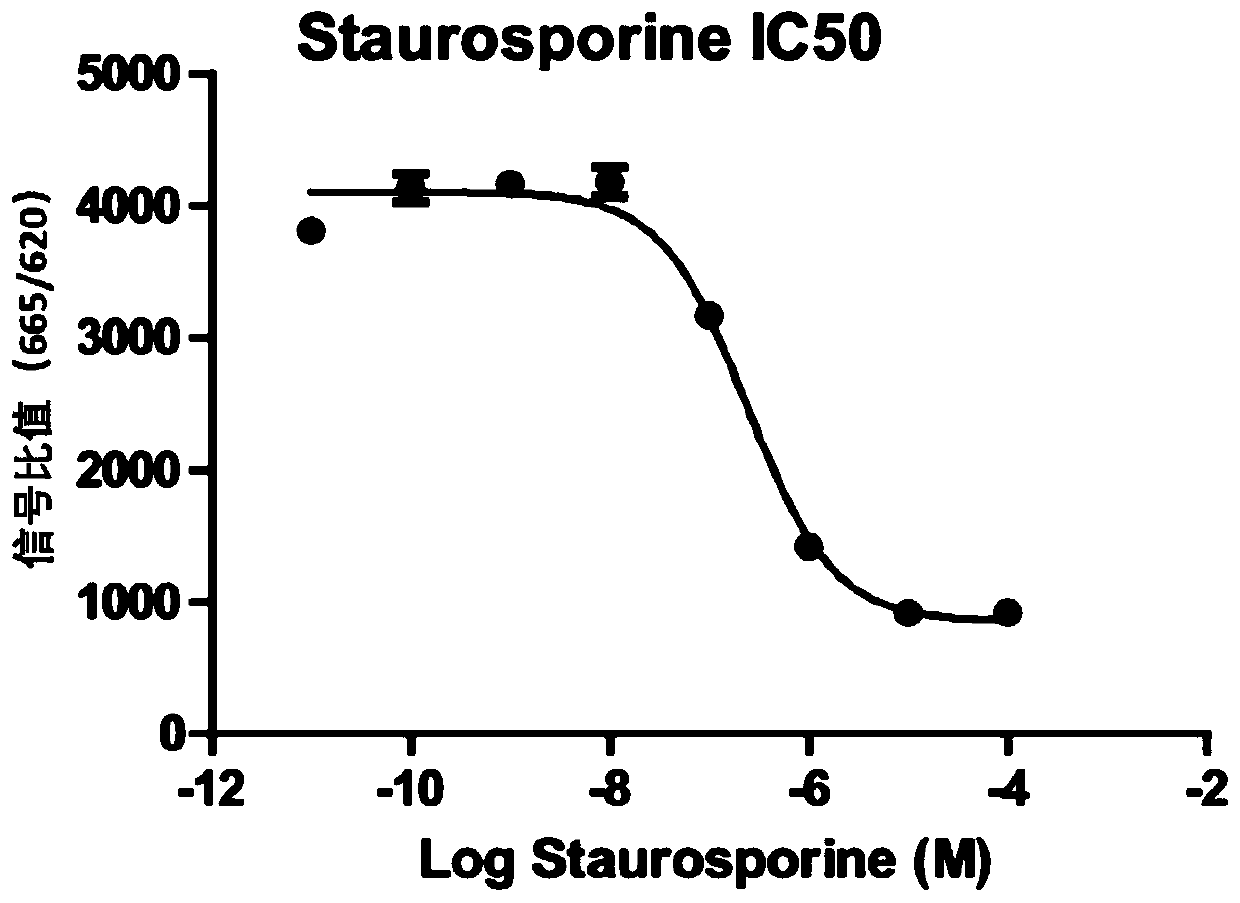
[0044] 1) Configure a positive compound, and perform a gradient dilution of the compound staurosporine, starting from 100000nM, and diluting it 10 times;
[0045] 2) Prepare EGFR, and perform follow-up inhibitor screening according to the appropriate kinase concentration of Example 1, EGFR is 0.0041ng / ul, and the enzyme reaction time is 40min;
[0046] 3) Add the prepared inhibitor, EGFR, substrate, and ATP to the 384-well detection plate in sequence, and after incubating at room temperature for 40 minutes, add the detection reagent coupled with Donor and Acceptor (or add 10ul after mixing in equal amounts), and incubate for 1 hour reading;
[0047] According to the principle of HTRF method, the fluorescence ratio of 665nm / 620nm is detected, and according to the ratio, the inhibition of the kinase is judged. The weaker the signal, the lower the kinase activity and the stronger the inhibitory effect. see results image 3 . image 3 The IC50 of compound Selleck A-1210477, AP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com