A composite iron carbodiimide battery negative electrode material and preparation method thereof
A technology for iron carbodiimide batteries and negative electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., to achieve the effects of improving conductivity and structural stability, enhancing reactivity, and easily obtaining raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Put 1g of ferric citrate and 1.5g of glucose into a mortar, mix and grind thoroughly, add 60mL of ethanol to the mixture A, put it into the reaction kettle and heat up to 200℃ at 10℃ / min in the homogeneous reactor After 24 hours, the obtained product was collected and centrifuged, centrifuged 4 times for 6 min each time, and the centrifuged product was dried at 60° C. to obtain process product B.
[0035]2) Mix 0.5g of process product B and 1g of urea in an alumina crucible, place the crucible in a tube furnace, and raise the temperature to 500°C at a constant rate of 10°C / min under an argon atmosphere. Solid phase reaction, after the reaction is completed, the reaction product FeNCN@C is collected after the entire reaction system is cooled.
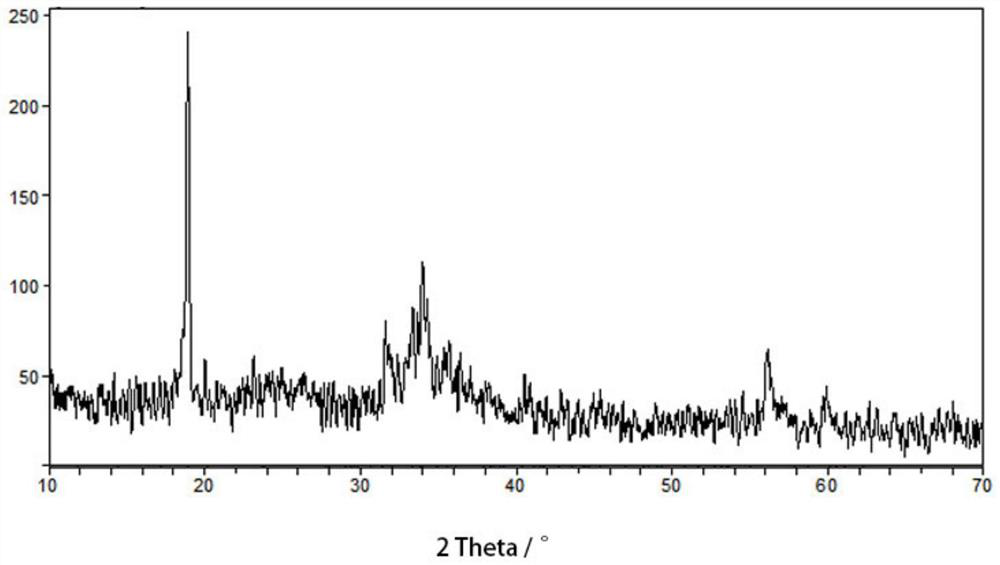
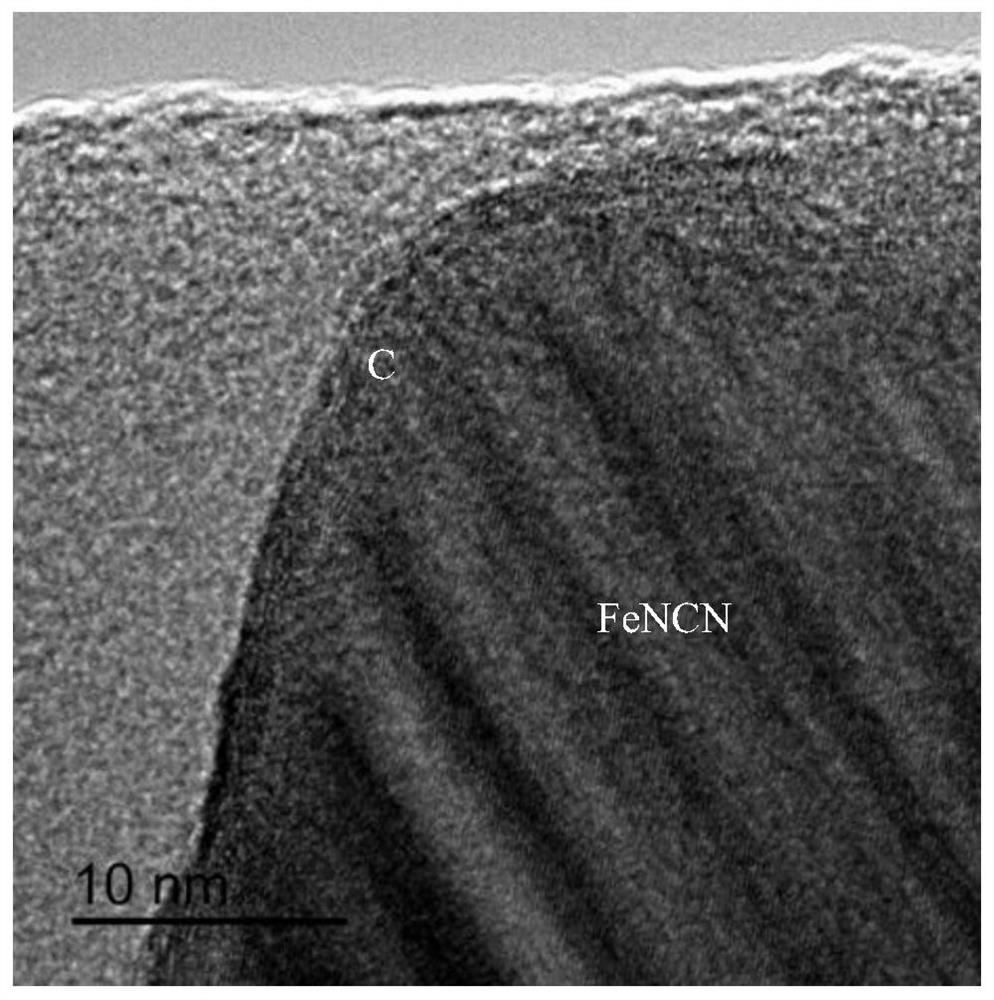
[0036] The product is analyzed by a Japanese Rigaku D / max2000PCX-ray diffractometer, and the XRD pattern of the reaction product FeNCN@C prepared in this example is as follows figure 1 The scanning electron microscope images o...
Embodiment 2
[0039] 1) Put 1g of ferric nitrate into a mortar, mix and grind it thoroughly with 2g of ammonium oxalate, add 60mL of ethanol to the mixture A, put it into the reaction kettle and raise the temperature to 200°C at 10°C / min in the homogeneous reactor for 12h , the obtained product was collected and centrifuged, centrifuged 4 times, each centrifuged for 6 min, and the centrifuged product was dried at 60° C. to obtain process product B.
[0040] 2) Mix 0.5g of process product B and 1.5g of urea into an alumina crucible, place the crucible in a tube furnace, and raise the temperature to 570°C at a constant rate of 20°C / min under an argon atmosphere. Carry out solid-state reaction, after the reaction is completed, the reaction product FeNCN@C is collected after the entire reaction system is cooled.
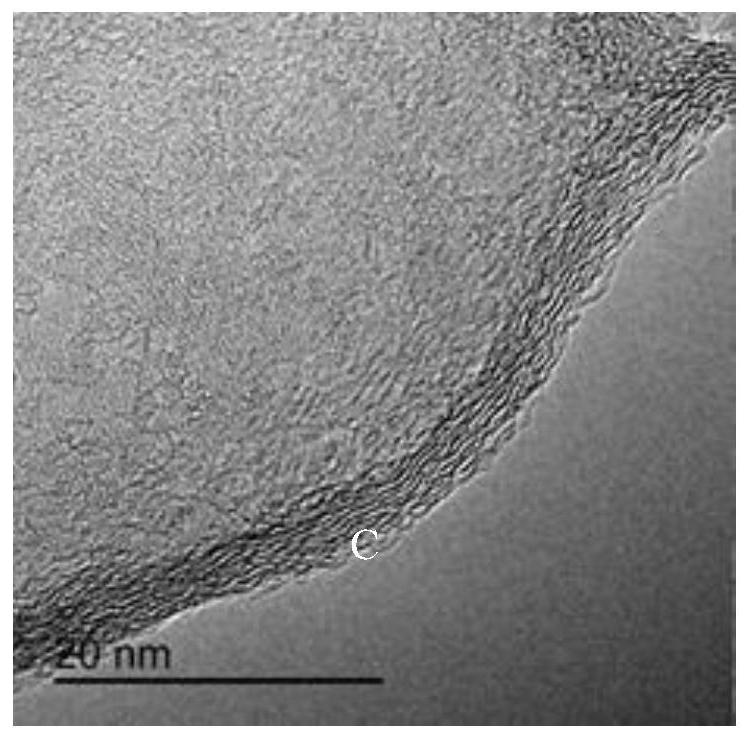
[0041] Adopt Japanese science D / max2000PCX-ray diffractometer to analyze product D, the XRD of gained product sees Figure 6 , the sample was observed under the scanning electron mic...
Embodiment 3
[0043] 1) Put 1g of ferric oxalate and 1g of sucrose into a mortar, mix and grind thoroughly, add 50mL of ethanol to the mixture A, put it into the reaction kettle, and raise the temperature to 200°C at 10°C / min in the homogeneous reactor for 36h. The obtained product was collected and centrifuged for 3 times, each centrifuged for 8 min, and the centrifuged product was dried at 65° C. to obtain process product B.
[0044] 2) Mix 1g of process product B and 3g of urea in an alumina crucible, place the crucible in a tube furnace, and raise the temperature to 300°C at a constant rate of 10°C / min under an argon atmosphere. After the reaction, the reaction product FeNCN@C was collected after the entire reaction system was cooled.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com